





Acta Veterinaria et Zootechnica Sinica ›› 2025, Vol. 56 ›› Issue (8): 4031-4041.doi: 10.11843/j.issn.0366-6964.2025.08.040
• Clinical Veterinary Medicine • Previous Articles Next Articles
ZHU Yixuan( ), GU Pengfei, ZHAO Qi, XU Panpan, FAN Yingsai, BAO Yongzhan, WANG Xiao*(
), GU Pengfei, ZHAO Qi, XU Panpan, FAN Yingsai, BAO Yongzhan, WANG Xiao*( ), SHI Wanyu*(
), SHI Wanyu*( )
)
Received:2024-10-23
Online:2025-08-23
Published:2025-08-28
Contact:
WANG Xiao, SHI Wanyu
E-mail:zyx1013583535@163.com;wxwangxiao418@163.com;shiwanyu2010@126.com
CLC Number:
ZHU Yixuan, GU Pengfei, ZHAO Qi, XU Panpan, FAN Yingsai, BAO Yongzhan, WANG Xiao, SHI Wanyu. Evaluation of PLGA Nanoparticles Co-loaded with Salvia miltiorrhiza Polysaccharide and Mn2+ as an Adjuvant for Inactivating PCV2[J]. Acta Veterinaria et Zootechnica Sinica, 2025, 56(8): 4031-4041.
Table 1
Flow cytometry antibody staining and cytokine detection methods"
| 时间 Time | 目标细胞 Target cells | 染色抗体[ Staining antibody | 细胞因子 Cytokine |
| 第21天 Day 21 | CD3+CD4+; CD3+CD8a+ | CD3-FITC; CD4-APC; CD8a-PE | 颗粒酶B |
| 细胞毒性T细胞 | CD8a-PE; CD107a(lamp-1)-FITC; CD178 FASL-APC | ||
| 第28天 Day 28 | 记忆T细胞 | CD4-APC;CD8a-PE;CD44-PE-Cyanine7;CD62L(L-Selectin)-FITC; | IFN-γ IFN-β IL-6 |
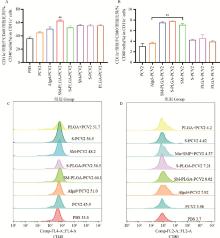
Fig. 1
Activation of DCs in lymph nodes A. The effect of each adjuvant group on the expression of CD40 on the surface of DCs; B. The effect of each adjuvant group on the expression of CD80 on the surface of DCs; C and D. Flow cytometry analysis of CD40 and CD80 expression in lymph node DCs. ** represents P < 0.01 compared with PCV2 group"
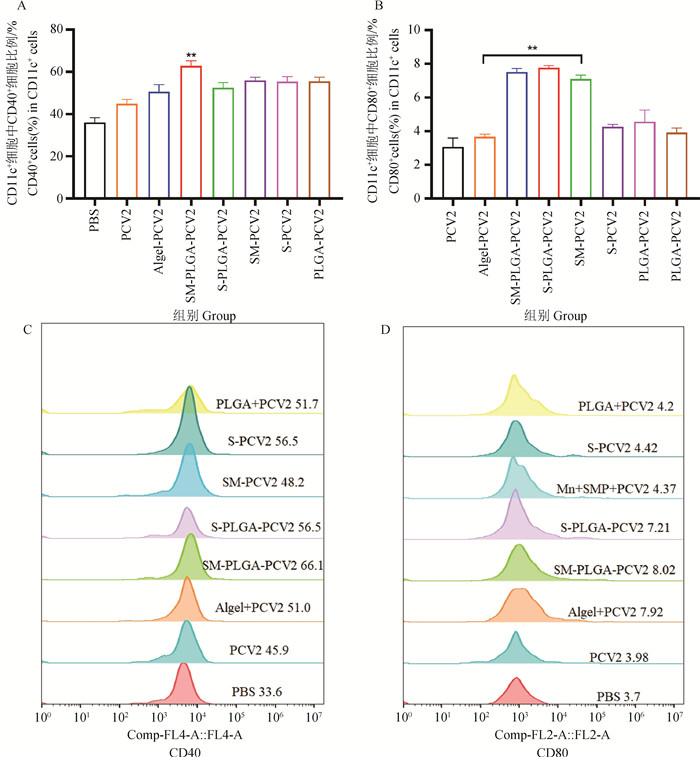
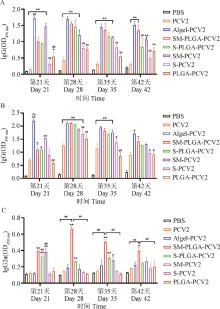
Fig. 2
Expression levels of PCV2-specific IgG and its subtypes in serum (n=3) A. The expression of PCV2 specific antibody IgG; B. The expression of PCV2 specific antibody IgG1; C. The expression of PCV2 specific antibody IgG2a; * represents P < 0.05 compared with PCV2 group, ** represents P < 0.01 compared with PCV2 group; # represents P < 0.05 compared with the SM-PLGA-PCV2 group, and ## represents P < 0.01 compared with the SM-PLGA-PCV2 group"
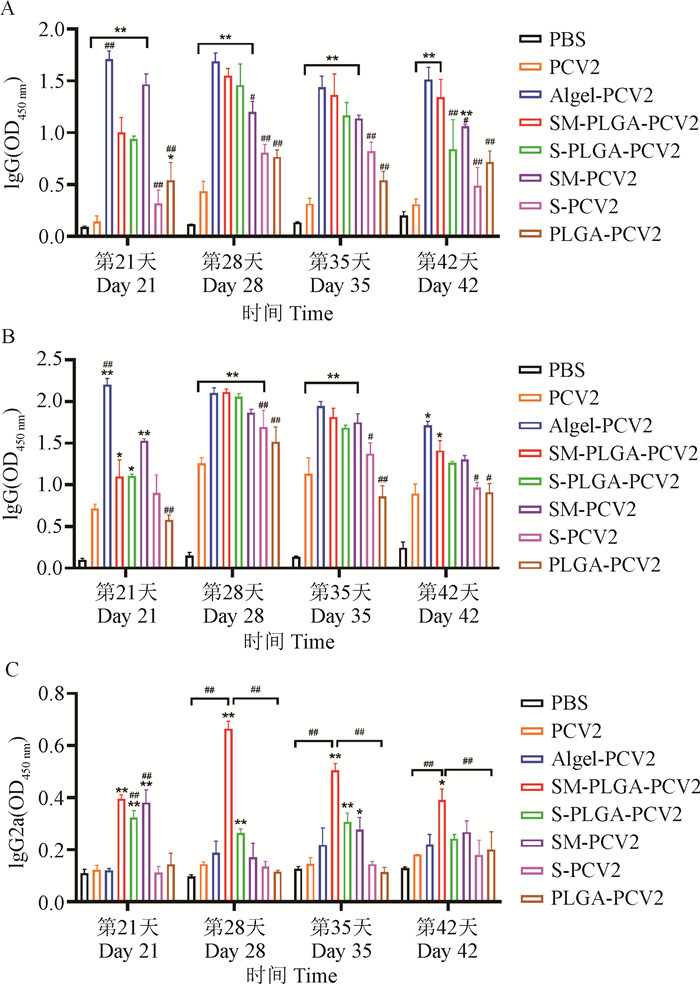
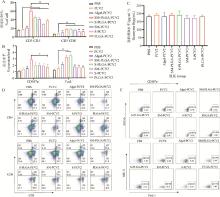
Fig. 3
Activation of spleen lymphocytes A. Activation of spleen CD4+ and CD8+ T lymphocytes; B. Activation of spleen CTL; C. The secretion of granzyme B; D. Flow scatter plot of CD4+ and CD8+ T lymphocytes; E. CTL flow scatter plot; * represents P < 0.05 compared with PCV2 group, ** represents P < 0.01 compared with PCV2 group; # represents P < 0.05 compared with the SM-PLGA-PCV2 group, and ## represents P < 0.01 compared with the SM-PLGA-PCV2 group."
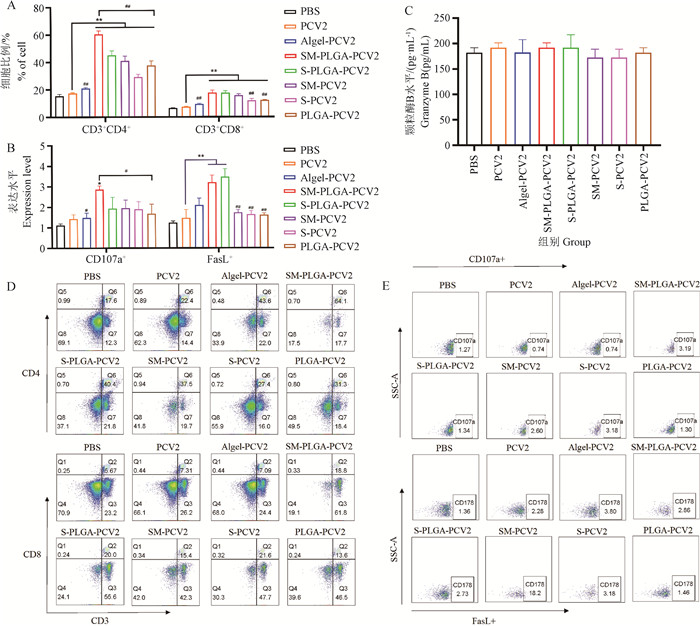
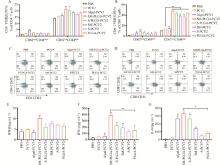
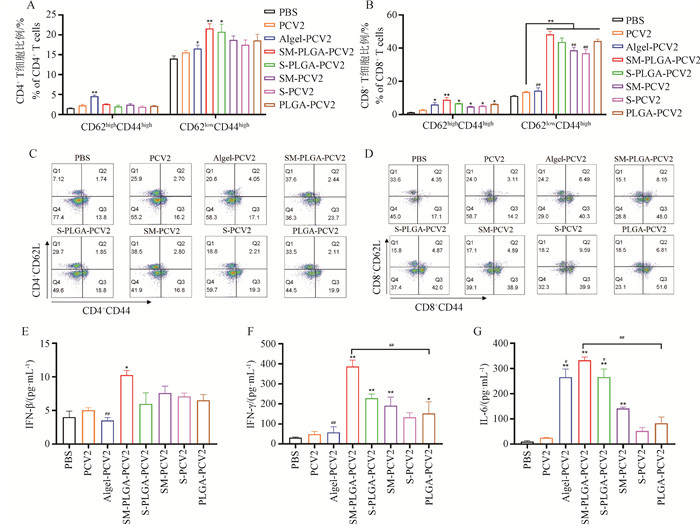
Fig. 4
Activation of spleen lymphocytes A. The activation of memory CD4+ T cells; B. Activation of memory CD8+ T cells; C. Memory CD4+ T cell flow scatter plot; D. Memory CD8+ T cell flow scatter plot; E. Secretion of IFN-β; F. Secretion of IFN-γ; G. Secretion of IL-6; * represents P < 0.05 compared with PCV2 group, ** represents P < 0.01 compared with PCV2 group; # represents P < 0.05 compared with the SM-PLGA-PCV2 group, and ## represents P < 0.01 compared with the SM-PLGA-PCV2 group"
| 1 |
FINSTERBUSCH T , MANKERTZ A . Porcine circoviruses--small but powerful[J]. Virus Res, 2009, 143 (2): 177- 183.
doi: 10.1016/j.virusres.2009.02.009 |
| 2 |
REN L , CHEN X , OUYANG H . Interactions of porcine circovirus 2 with its hosts[J]. Virus Genes, 2016, 52 (4): 437- 444.
doi: 10.1007/s11262-016-1326-x |
| 3 |
SEGALÉS J . Porcine circovirus type 2 (PCV2) infections: clinical signs, pathology and laboratory diagnosis[J]. Virus Res, 2012, 164 (1-2): 10- 19.
doi: 10.1016/j.virusres.2011.10.007 |
| 4 |
LIU X , OUYANG T , MA T , et al. Immunogenicity evaluation of inactivated virus and purified proteins of porcine circovirus type 2 in mice[J]. BMC Vet Res, 2018, 14 (1): 137.
doi: 10.1186/s12917-018-1461-9 |
| 5 |
CHAE C . A review of porcine circovirus 2-associated syndromes and diseases[J]. Vet J, 2005, 169 (3): 326- 336.
doi: 10.1016/j.tvjl.2004.01.012 |
| 6 |
HUANG Y , LIU Z , BO R , et al. The enhanced immune response of PCV-2 vaccine using Rehmannia glutinosa polysaccharide liposome as an adjuvant[J]. Int J Biol Macromol, 2016, 86, 929- 936.
doi: 10.1016/j.ijbiomac.2016.02.003 |
| 7 |
ZHANG G , JIA P , CHENG G , et al. Enhanced immune response to inactivated porcine circovirus type 2 (PCV2) vaccine by conjugation of chitosan oligosaccharides[J]. Carbohydr Polym, 2017, 166, 64- 72.
doi: 10.1016/j.carbpol.2017.02.058 |
| 8 | 邓盈盈. 猪圆环病毒2型Cap蛋白抗独特型纳米抗体免疫保护性及其机制研究[D]. 杨凌: 西北农林科技大学, 2023. |
| DENG Y Y. Study on the immune protection and mechanism of porcine circovirus type 2 Cap protein anti-idiotypic nanobody[D]. Yangling: Northwest A&F University, 2023. (in Chinese) | |
| 9 |
SUN J , HUANG L , WEI Y , et al. Prevalence of emerging porcine parvoviruses and their co-infections with porcine circovirus type 2 in China[J]. Arch Virol, 2015, 160 (5): 1339- 1344.
doi: 10.1007/s00705-015-2373-7 |
| 10 |
BEACH N M , MENG X-J . Efficacy and future prospects of commercially available and experimental vaccines against porcine circovirus type 2 (PCV2)[J]. Virus Res, 2012, 164 (1-2): 33- 42.
doi: 10.1016/j.virusres.2011.09.041 |
| 11 |
BURAKOVA Y , MADERA R , MCVEY S , et al. Adjuvants for animal vaccines[J]. Viral Immunol, 2018, 31 (1): 11- 22.
doi: 10.1089/vim.2017.0049 |
| 12 |
EXLEY C . Aluminium adjuvants and adverse events in sub-cutaneous allergy immunotherapy[J]. Allergy Asthma Clin Immunol, 2014, 10 (1): 4.
doi: 10.1186/1710-1492-10-4 |
| 13 |
LIU Z , XING J , HUANG Y , et al. Activation effect of Ganoderma lucidum polysaccharides liposomes on murine peritoneal macrophages[J]. Int J Biol Macromol, 2016, 82, 973- 978.
doi: 10.1016/j.ijbiomac.2015.10.088 |
| 14 |
WAN X , YIN Y , ZHOU C , et al. Polysaccharides derived from Chinese medicinal herbs: A promising choice of vaccine adjuvants[J]. Carbohydr Polym, 2022, 276, 118739.
doi: 10.1016/j.carbpol.2021.118739 |
| 15 |
ZHU Y , YANG X , GU P , et al. The structural characterization of a polysaccharide from the dried root of Salvia miltiorrhiza and its use as a vaccine adjuvant to induce humoral and cellular immune responses[J]. Int J Mol Sci, 2024, 25 (14): 7765.
doi: 10.3390/ijms25147765 |
| 16 |
JIANG Y , WANG L , ZHANG L , et al. Optimization of extraction and antioxidant activity of polysaccharides from Salvia miltiorrhiza Bunge residue[J]. Int J Biol Macromol, 2015, 79, 533- 541.
doi: 10.1016/j.ijbiomac.2015.05.024 |
| 17 |
JI H Y , LIU C , DAI K Y , et al. The extraction, structure, and immunomodulation activities in vivo of polysaccharides from Salvia miltiorrhiza[J]. Ind Crop Prod, 2021, 173, 114085.
doi: 10.1016/j.indcrop.2021.114085 |
| 18 |
LATZ E , XIAO T S , STUTZ A . Activation and regulation of the inflammasomes[J]. Nat Rev Immunol, 2013, 13 (6): 397- 411.
doi: 10.1038/nri3452 |
| 19 |
CHEN Y , LI H , LI M , et al. Salvia miltiorrhiza polysaccharide activates T lymphocytes of cancer patients through activation of TLRs mediated -MAPK and -NF-κB signaling pathways[J]. J Ethnopharmacol, 2017, 200, 165- 173.
doi: 10.1016/j.jep.2017.02.029 |
| 20 |
WANG N , YANG J , LU J , et al. A polysaccharide from Salvia miltiorrhiza Bunge improves immune function in gastric cancer rats[J]. Carbohydr Polym, 2014, 111, 47- 55.
doi: 10.1016/j.carbpol.2014.04.061 |
| 21 |
WANG C , GUAN Y , LV M , et al. Manganese increases the sensitivity of the cGAS-STING pathway for double-stranded DNA and is required for the host defense against DNA viruses[J]. Immunity, 2018, 48 (4): 675- 687.
doi: 10.1016/j.immuni.2018.03.017 |
| 22 |
PORANEN M M , SALGADO P S , KOIVUNEN M R L , et al. Structural explanation for the role of Mn2+ in the activity of phi6 RNA-dependent RNA polymerase[J]. Nucleic Acids Res, 2008, 36 (20): 6633- 6644.
doi: 10.1093/nar/gkn632 |
| 23 |
ZHAO Z , MA Z , WANG B , et al. Mn2+ directly activates cGAS and structural analysis suggests Mn2+ induces a noncanonical catalytic synthesis of 2'3'-cGAMP[J]. Cell Rep, 2020, 32 (7): 108053.
doi: 10.1016/j.celrep.2020.108053 |
| 24 |
LIU Z , XING J , ZHENG S , et al. Ganoderma lucidum polysaccharides encapsulated in liposome as an adjuvant to promote Th1-bias immune response[J]. Carbohydr Polym, 2016, 142, 141- 148.
doi: 10.1016/j.carbpol.2016.01.021 |
| 25 |
ZHANG S , PANG G , CHEN C , et al. Effective cancer immunotherapy by Ganoderma lucidum polysaccharide-gold nanocomposites through dendritic cell activation and memory T cell response[J]. Carbohydr Polym, 2019, 205, 192- 202.
doi: 10.1016/j.carbpol.2018.10.028 |
| 26 |
SARTI F , PERERA G , HINTZEN F , et al. In vivo evidence of oral vaccination with PLGA nanoparticles containing the immunostimulant monophosphoryl lipid A[J]. Biomaterials, 2011, 32 (16): 4052- 4057.
doi: 10.1016/j.biomaterials.2011.02.011 |
| 27 | 张威风. 粒径均一聚乳酸基微球作为亚单位疫苗佐剂的研究[D]. 北京: 中国科学院研究生院, 2015. |
| ZHANG W F. Study on uniform particle size polylactic acid microspheres as adjuvants for subunit vaccines[D]. Beijing: Graduate School of Chinese Academy of Sciences, 2015. (in Chinese) | |
| 28 |
ZHU Y X , ZHAO Q , GU P F , et al. PLGA co-loaded Salvia miltiorrhiza polysaccharide and Mn2+ as an adjuvant to induce potent immunity[J]. Int J Biol Macromol, 2025, 300, 140050.
doi: 10.1016/j.ijbiomac.2025.140050 |
| 29 | 谷鹏飞. 聚乙烯亚胺修饰的当归多糖PLGA纳米粒的佐剂活性及作用机理研究[D]. 南京: 南京农业大学, 2022. |
| GU P F. Study on the adjuvant activity and mechanism of polyethyleneimine-coated PLGA nanoparticles-encapsulated angelica sinensis polysaccharide[D]. Nanjing: Nanjing Agricultural University, 2022. (in Chinese) | |
| 30 |
WANG Z , YUAN Y , CHEN C , et al. Colloidal manganese salt improves the efficacy of rabies vaccines in mice, cats, and dogs[J]. J Virol, 2021, 95 (23): e0141421.
doi: 10.1128/JVI.01414-21 |
| 31 |
LU T , HU F , YUE H , et al. The incorporation of cationic property and immunopotentiator in poly (lactic acid) microparticles promoted the immune response against chronic hepatitis B[J]. J Control Release, 2020, 321, 576- 588.
doi: 10.1016/j.jconrel.2020.02.039 |
| 32 |
ZHANG W , WANG L , LIU Y , et al. Immune responses to vaccines involving a combined antigen-nanoparticle mixture and nanoparticle-encapsulated antigen formulation[J]. Biomaterials, 2014, 35 (23): 6086- 6097.
doi: 10.1016/j.biomaterials.2014.04.022 |
| 33 | SABJAN K B , MUNAWAR S M , RAJENDIRAN D , et al. Nanoemulsion as oral drug delivery-a review[J]. Curr Drug Res Rev, 2020, 12 (1): 14- 15. |
| 34 |
DU G , HATHOUT R M , NASR M , et al. Intradermal vaccination with hollow microneedles: A comparative study of various protein antigen and adjuvant encapsulated nanoparticles[J]. J Control Release, 2017, 266, 109- 118.
doi: 10.1016/j.jconrel.2017.09.021 |
| 35 |
TRAPANI J A , SMYTH M J . Functional significance of the perforin/granzyme cell death pathway[J]. Nat Rev Immunol, 2002, 2 (10): 735- 747.
doi: 10.1038/nri911 |
| 36 |
LEE J Y , CHAE D W , KIM S M , et al. Expression of FasL and perforin/granzyme B mRNA in chronic hepatitis B virus infection[J]. J Viral Hepat, 2004, 11 (2): 130- 135.
doi: 10.1046/j.1365-2893.2003.00486.x |
| 37 |
HU F , YUE H , LU T , et al. Cytosolic delivery of HBsAg and enhanced cellular immunity by pH-responsive liposome[J]. J Control Release, 2020, 324, 460- 470.
doi: 10.1016/j.jconrel.2020.05.042 |
| 38 |
JIA J , LIU Q , YANG T , et al. Facile fabrication of varisized calcium carbonate microspheres as vaccine adjuvants[J]. J Mater Chem B, 2017, 5 (8): 1611- 1623.
doi: 10.1039/C6TB02845D |
| 39 | 卫潇茗, 王晨光, 张睿, 等. 锰离子作为免疫调节剂的发现及应用展望[J]. 中国细胞生物学学报, 2020, 42 (10): 1721- 1731. |
| WEI X M , WANG C G , ZHANG R , et al. The discovery and application of manganese ions as immunomodulators[J]. Chinese Journal of Cell Biology, 2020, 42 (10): 1721- 1731. | |
| 40 |
KUMAR S , KESHARWANI S S , KUPPAST B , et al. Pathogen-mimicking vaccine delivery system designed with a bioactive polymer (inulin acetate) for robust humoral and cellular immune responses[J]. J Control Release, 2017, 261, 263- 274.
doi: 10.1016/j.jconrel.2017.06.026 |
| 41 |
COURANT T , BAYON E , REYNAUD-DOUGIER H L , et al. Tailoring nanostructured lipid carriers for the delivery of protein antigens: Physicochemical properties versus immunogenicity studies[J]. Biomaterials, 2017, 136, 29- 42.
doi: 10.1016/j.biomaterials.2017.05.001 |
| 42 |
CHEN X , LIU Y , WANG L , et al. Enhanced humoral and cell-mediated immune responses generated by cationic polymer-coated PLA microspheres with adsorbed HBsAg[J]. Mol Pharm, 2014, 11 (6): 1772- 1784.
doi: 10.1021/mp400597z |
| 43 |
LIU Y , JIAO F , QIU Y , et al. The effect of Gd@C82(OH)22 nanoparticles on the release of Th1/Th2 cytokines and induction of TNF-alpha mediated cellular immunity[J]. Biomaterials, 2009, 30 (23-24): 3934- 3945.
doi: 10.1016/j.biomaterials.2009.04.001 |
| 44 |
QIAO N , WANG H , XU Y , et al. A MnAl double adjuvant nanovaccine to induce strong humoral and cellular immune responses[J]. J Control Release, 2023, 358, 190- 203.
doi: 10.1016/j.jconrel.2023.04.036 |
| 45 | 卢婷. 基于聚乳酸微球佐剂的乙肝疫苗研究[D]. 北京: 中国科学院大学, 2020. |
| LU T. Study on hepatitis B vaccine based on polylactic acid microsphere adjuvant[D]. Beijing: University of Chinese Academy of Sciences, 2020. (in Chinese) |
| [1] | LIN Xinyi, JIANG Xinyu, SU Zinuo, WANG Yuling, RUAN Shiyu, HONG Hailong, WU Jiahao, BO Ruonan. Study on Preparation and the Mucosal Immune Adjuvant Activity of Ultra-Large Mesoporous Silica Nanoparticles Loading Polysaccharide from Atractylodes macrocephala Koidz [J]. Acta Veterinaria et Zootechnica Sinica, 2025, 56(5): 2507-2519. |
| [2] | Mengdi WANG, Heng WANG, Xiuxiang LU, Yumin WANG, Wenjie FAN, Chen YAO, Pengxiang LIU, Yanjie MA, Guoyu YANG. Preparation of Nano-manganese and Its Biological Effects [J]. Acta Veterinaria et Zootechnica Sinica, 2024, 55(8): 3374-3382. |
| [3] | ZHOU Mengting, SONG Yinjuan, XU Jian, LI Bin, RAN Duoliang, CHU Yuefeng. Advances in Carbohydrate-based Adjuvant Mechanisms of Action [J]. Acta Veterinaria et Zootechnica Sinica, 2024, 55(2): 491-501. |
| [4] | WANG Zi, WANG Nianxiang, TIAN Changming, ZHAO Fujie, LIU Lintao, MA Mengyao, JIA Xinhao, LIU Guoxing, ZHENG Lanlan. Using Mouse to Evaluate the Immune Effect of Bridged Diphenylalanine Dipeptide with Inactivated Porcine Deltacoronavirus [J]. Acta Veterinaria et Zootechnica Sinica, 2023, 54(4): 1590-1597. |
| [5] | LI Rang, WENG Xiang, LI Quanxiao, WU Daocheng, CAO Hui, ZHANG Ailian. Analysis of Emulsifying Method and Stability of Foot-and-mouth Disease Vaccine Combined with Crude Polysaccharides from Cultivated Artemisia rupestris L. [J]. Acta Veterinaria et Zootechnica Sinica, 2023, 54(4): 1608-1615. |
| [6] | PAN Weixiong, CHEN Yuyi, ZHAO Zengjue, FENG Saixiang, QIU Weihong, YE Hejia, ZHANG Linghua. Rhodococcus ruber Synergizes Effectively with CpG ODN to Enhance the Immune Efficacy of the Bird Flu Vaccine in Chickens [J]. Acta Veterinaria et Zootechnica Sinica, 2022, 53(12): 4367-4378. |
| [7] | YIMAMU·Ruziwanguli, ZHANG Ailian, WENG Xiang, XIAO Peng, CAO Hui, WU Daocheng. Comparison of Adjuvant Activity Between Xinjiang Cultivated/Wild Artemisia rupestris L. Crude Polysaccharides on Foot-and-mouth Disease Vaccine in Mice [J]. Acta Veterinaria et Zootechnica Sinica, 2022, 53(11): 4089-4096. |
| [8] | WENG Xiang, ZHANG Ailian, LI Quanxiao, WU Daocheng, CAO Hui. Immuno-enhancement Effects of Cultivated Artemisia rupestris L. Crude Polysaccharide on Foot-and-mouth Disease Vaccine via Subcutaneous Route [J]. Acta Veterinaria et Zootechnica Sinica, 2022, 53(1): 315-323. |
| [9] | LI Xian, ZHANG Zhongwang, ZHANG Fudong, Lü Jianliang, LI Jiahao, PAN Li. Evaluation of the Characteristics of Mannosylated Chitosan PLGA Nanospheres as a Delivery System for DNA Vaccine of A/FMDV [J]. Acta Veterinaria et Zootechnica Sinica, 2021, 52(12): 3557-3568. |
| [10] | MENG Zhen, SUN Mengke, XU Yongde, QIN Tao, REN Zhe. Structural Characterization and Immune Enhancement of Phyllanthus emblica Polysaccharide [J]. Acta Veterinaria et Zootechnica Sinica, 2021, 52(12): 3627-3640. |
| [11] | LIU Danhua, ZHENG Shimin, LIU Xiaojing, Lü Xiaoping, GAO Xueli, LIU Chaonan. Effects of Avian Reticuloendotheliosis Virus Infection on the CD4+/CD8+ Ratio and the Expression of Related Cytokines in SPF Chicks [J]. Acta Veterinaria et Zootechnica Sinica, 2020, 51(6): 1447-1454. |
| [12] | PEI Shixuan, SONG Jijian, HAN Yinong, XUE Yun, WANG Chen, SI Lifang, ZHAO Zhanqin. Comparison of Adjuvant for Inactivated Swine Erysipelas Vaccines against Erysipelothrix rhusiopathiae [J]. Acta Veterinaria et Zootechnica Sinica, 2020, 51(5): 1083-1090. |
| [13] | YAO Yan-bin, CHEN Zhang, CHU Xia-fei, WEI Jian-zhong, SUN Pei, LI Yu. Immune Efficacy of Transferrin-binding Protein A of Haemophilus parasuis in Piglets [J]. ACTA VETERINARIA ET ZOOTECHNICA SINICA, 2018, 49(3): 588-596. |
| [14] | TAN Xin, KU Xu-gang, YU Xue-xiang, GUO Heng-ke, HE Dong-xian, FAN Sheng-xian, HE Qi-gai. The IFN-γ-ELISpot for Evaluating the Immune Effect of Swine Pseudorabies Vaccine [J]. ACTA VETERINARIA ET ZOOTECHNICA SINICA, 2017, 48(10): 1939-1948. |
| [15] | ZHOU Ye-fei,ZHOU Mei-xian. Gluten Exorphin B5 Enhances Immunogenicity and Protective Efficacy of Infectious Bronchitis Virus Vaccine in Broilers [J]. ACTA VETERINARIA ET ZOOTECHNICA SINICA, 2016, 47(3): 566-583. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||