





Acta Veterinaria et Zootechnica Sinica ›› 2026, Vol. 57 ›› Issue (1): 513-525.doi: 10.11843/j.issn.0366-6964.2026.01.045
• CLINICAL VETERINARY MEDICINE • Previous Articles Next Articles
WEN Xin1,2,3,4( ), LI Yongshuai1,2,3,4(
), LI Yongshuai1,2,3,4( ), WANG Luyu1, ZHAO Yan1,2,3,4, SUN Jingwen1,2,3,4, SHI Huijun1,2,3,4, FU Qiang1,2,3,4, YANG Li1,2,3,4(
), WANG Luyu1, ZHAO Yan1,2,3,4, SUN Jingwen1,2,3,4, SHI Huijun1,2,3,4, FU Qiang1,2,3,4, YANG Li1,2,3,4( )
)
Received:2024-12-23
Online:2026-01-23
Published:2026-01-26
Contact:
YANG Li
E-mail:1090798346@qq.com;1485447034@qq.com;563289492@qq.com
CLC Number:
WEN Xin, LI Yongshuai, WANG Luyu, ZHAO Yan, SUN Jingwen, SHI Huijun, FU Qiang, YANG Li. To Analyze the Mechanism of Berberine against Staphylococcus aureus Infection Based on Network Pharmacology and Experimental Verification[J]. Acta Veterinaria et Zootechnica Sinica, 2026, 57(1): 513-525.
Table1
Primer sequence"
基因 Gene | 登录号 GenBank ID | 引物序列(5'→3') Primer sequence | 产物长度/bp Product length |
|---|---|---|---|
| Jun-F | NM_010591.2 | GCACATCACCACTACACCGA | 127 |
| Jun-R | GGGAAGCGTGTTCTGGCTAT | ||
| Cdkn1a-F | NM_007669.5 | AGTGTGCCGTTGTCTCTTCG | 147 |
| Cdkn1a-R | CAGACGAAGTTGCCCTCCAG | ||
| Esr1-F | NM_001302531.1 | GGTGCCCTACTACCTGGAGA | 188 |
| Esr1-R | CATTGCACACGGCACAGTAG | ||
| Src-F | NM_001025395.3 | CGGTTACATCCCCAGCAACT | 198 |
| Src-R | TTGGCATTGTCGAAGTCGGA | ||
| Trp53-F | NM_001127233.1 | TGGAGGAGTCACAGTCGGAT | 117 |
| Trp53-R | CGTCCATGCAGTGAGGTGAT | ||
| Casp3-F | NM_001284409.1 | CTTCCATAAGAGCACTGGAATGT | 183 |
| Casp3-R | ACAAAGCTGCTCCTTTTGCTATG | ||
| GAPDH-F | NM_002046 | GAAGGTCGGTGTGAACGGAT | 233 |
| GAPDH-R | CTCGCTCCTGGAAGATGGTG |
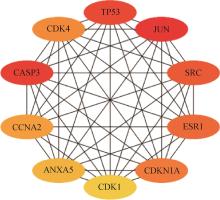
Fig.5
PPI core target network diagramRed nodes. Potentially key regulatory proteins or molecules that play a central role in the onset and progression of disease. Red nodes in the figure include JUN, CASP3, SRC, and ESR1. Orange nodes. These nodes represent targets directly connected to red nodes. They may be slightly less important than red nodes in the network, but they are still important interaction partners. In the figure, orange nodes include TP53, CDK4, CCNA2, and CDKN1A. Yellow nodes. These nodes may represent other important targets in the network, potentially playing roles in specific biological processes or having indirect interactions with red and orange nodes. In the figure, yellow nodes include ANXA5, CDK1, and CDKN1A"

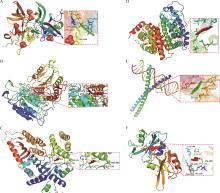
Fig. 6
Molecular docking resultsA. Hydrophobic interactions between BBR molecules and Tp53 protein. B. Hydrophobic interactions between BBR molecules and Casp3 protein. C. Hydrophobic interactions between BBR molecules and Cdkn1a protein. D. Hydrophobic interactions between BBR molecules and Esr1 protein. E. Hydrophobic interactions between BBR molecules and Jun protein. F. Hydrophobic interactions between BBR molecules and Src protein."

Table 3
Body mass and organ index of mice (x±s)"
组别 Group | 初始体重/g Initial weight | 攻毒前体重/g Weight before challenge | 最终体重/g Final weight | 肝指数/% Hepatic index | 脾指数/% Spleen index | 肾指数/% Renal index |
|---|---|---|---|---|---|---|
| NC | 19.22±0.16 | 20.52±1.16 | 23.20±1.99 | 6.12±0.39 | 0.29±0.03 | 1.22±0.07 |
| MC | 20.60±0.22 | 21.49±1.16 | 19.90±1.59 | 6.52±0.45 | 0.33±0.11 | 1.23±0.04 |
| BBR-MC | 21.06±1.10 | 21.32±0.70 | 19.96±1.28 | 6.28±0.79 | 0.24±0.03 | 1.21±0.09 |
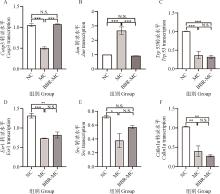
Fig.8
Transcription quantity of core target genes in mice liver*. P<0.05, indicates a statistically significant difference; **. P<0.01, indicates a more pronounced difference; ***. P<0.001, indicates a significant difference; ****. P<0.001, indicates a highly significant difference; NS. Denotes non-significant. The same as below"

| [1] | 陈 云,杨 峰,李新圃,等.牛源耐甲氧西林金黄色葡萄球菌研究进展[J].动物医学进展,2019,40(11):104-109. |
| CHEN Y,YANG F,LI X P,et al.Research progress on bovine-derived methicillin-resistant Staphylococcus aureus [J].Progress in Veterinary Medicine,2019,40(11):104-109.(in Chinese) | |
| [2] | HEINZINGER L R,PUGH A R,WAGNER J A,et al.Evaluating the translational potential of bacteriocins as an alternative treatment for Staphylococcus aureus infections in animals and humans[J].Antibiotics (Basel),2023,12(8):1256. |
| [3] | 戚颖欣.五味子酮抑制金黄色葡萄球菌α-溶血素的表达及作用机制研究[D].长春:吉林农业大学,2023. |
| QI Y X.Study on the inhibitory effect and mechanism of schisandrin on α-hemolysin expression in Staphylococcus aureus[D].Changchun:Jilin Agricultural University,2023.(in Chinese) | |
| [4] | GUO Y,SONG G,SUN M,et al.Prevalence and therapies of antibiotic-resistance in Staphylococcus aureus[J].Front Cell Infect Microbiol,2020,10:107. |
| [5] | 屈 欢,黄 雪,王军节.黄连中盐酸小檗碱的提取纯化及抑菌活性研究[J].植物保护,2020,46(02):96-100. |
| QU H,HUANG X,WANG J J.Extraction,purification,and antibacterial activity of berberine hydrochloride from Coptis chinensis[J].Plant Protection,2020,46(02):96-100.(in Chinese) | |
| [6] | QI Y,ZHANG Q,ZHU H.Huang-Lian Jie-Du decoction:a review on phytochemical,pharmacological and pharmacokinetic investigations[J].Chin Med,2019,14:57. |
| [7] | 孟治平.盐酸小檗碱吸入制剂的制备及其治疗呼吸道金黄色葡萄球菌感染的研究[D].南京:南京中医药大学,2021. |
| MENG Z P.Preparation of berberine hydrochloride inhalation formulation and its therapeutic effect on respiratory tract infection caused by Staphylococcus aureus[D].Nanjing:Nanjing University of Chinese Medicine,2021.(in Chinese) | |
| [8] | 张旭梅,魏玉荣,许丞惠,等.基于网络药理学和试验验证分析小檗碱治疗鸡沙门菌感染的作用机制[J].畜牧兽医学报,2023,54(8):3557-3570. |
| ZHANG X M,WEI Y R,XU C H,et al.Network pharmacology and experimental validation of berberine in treating Salmonella infection in chickens[J].Acta Veterinaria et Zootechnica Sinica,2023,54(08):3557-3570.(in Chinese) | |
| [9] | 曹启航.单核细胞增生李斯特菌生物被膜形成过程的转录组学分析[D].兰州:甘肃农业大学,2021. |
| CAO Q H.Transcriptomic analysis of biofilm formation in Listeria monocytogenes[D].Lanzhou:Gansu Agricultural University,2021.(in Chinese) | |
| [10] | 邓礼娟,黄洁瑶,胡彦君,等.小檗碱对糖尿病肾病小鼠肾脏中 FXR 和 SHP 表达的影响[J].中国药理学通报,2024,40 (12):2269-2276. |
| DENG L J,HUANG J Y,HU Y J,et al.Berberine affects the expression of FXR and SHP in the kidneys of mice with diabetic nephropathy[J].Chinese Pharmacological Bulletin,2024,40(12):2269-2276.(in Chinese) | |
| [11] | KRISHNAMOORTHY P,SATYANARAYANA M L,SHOME B R,et al.Pathological changes in experimental intramammary infection with different Staphylococcus species in mice[J].J Microsc Ultrastruct,2018,6(2):93-98. |
| [12] | BARTSCH P,KILIAN C,HELLMIG M,et al.Th17 cell plasticity towards a T-bet-dependent Th1 phenotype is required for bacterial control in Staphylococcus aureus infection[J].PLoS Pathog,2022,18(4):e1010430. |
| [13] | TILAHUN A Y,HOLZ M,WU T T,et al.Interferon gamma-dependent intestinal pathology contributes to the lethality in bacterial superantigen-induced toxic shock syndrome[J].PLoS One,2011,6(2):e16764. |
| [14] | 罗 阳,田唯佳,何 芳,等.奶牛乳房炎金黄色葡萄球菌nuc基因LAMP检测方法的建立[J].动物医学进展.2023,44(8):34-38. |
| LUO Y,TIAN W J,HE F,et al.Establishment of LAMP method for detecting nuc gene in Staphylococcus aureus from bovine mastitis[J].Progress in Veterinary Medicine,2023,44(08):34-38.(in Chinese) | |
| [15] | 唐东涛,王添昊,芈聪慧,等.羟丙基甲基纤维素基涂膜的制备及香蕉保鲜效果[J].食品工业科技,2023,44(12):268-275. |
| TANG D T,WANG T H,MI C H,et al.Preparation of hydroxypropyl methylcellulose-based coating and its preservation effect on bananas[J].Science and Technology of Food Industry,2023,44(12):268-275.(in Chinese) | |
| [16] | 王 礼.小檗碱抗金黄色葡萄球菌的机制研究[D].兰州:兰州大学,2018. |
| WANG L.Mechanism of berberine against Staphylococcus aureus[D].Lanzhou:Lanzhou University,2018.(in Chinese) | |
| [17] | WU S,YANG K,HONG Y,et al.A new perspective on the antimicrobial mechanism of berberine hydrochloride against Staphylococcus aureus revealed by untargeted metabolomic studies[J].Front Microbiol,2022,13:917414. |
| [18] | QIU F,LU W,YE S,et al.Berberine promotes induction of immunological tolerance to an allograft via downregulating memory CD8+ T-cells through altering the gut microbiota[J].Front Immunol,2021,12:646831. |
| [19] | 黄启发.肿瘤内痤疮丙酸杆菌通过调节Hh信号通路促进卵巢癌进展[D].南昌:南昌大学,2023. |
| HUANG Q F.Intratumoral Propionibacterium acnes promotes ovarian cancer progression via Hedgehog signaling pathway[D].Nanchang:Nanchang University,2023.(in Chinese) | |
| [20] | 武明云,虞坚尔,薛 征,等.基于MAPK信号通路的中药治疗支气管哮喘的实验研究进展[J].上海中医药大学学报,2019,33(2):86-91. |
| WU M Y,YU J E,XUE Z,et al.Research progress on experimental study of traditional Chinese medicine treating bronchial asthma based on MAPK signaling pathway[J].Acta Universitatis Traditionis Medicalis Sinensis Pharmacologiaeque Shanghai,2019,33(2):86-91.(in Chinese) | |
| [21] | 李 帅.金黄色葡萄球菌通过TLR2/PI3K信号通路诱导巨噬细胞自噬和炎症反应的分子机制研究[D].合肥:安徽医科大学,2018. |
| LI S.Molecular mechanism of Staphylococcus aureus-induced macrophage autophagy and inflammation via TLR2/PI3K signaling pathway[D].Hefei:Anhui Medical University,2018.(in Chinese) | |
| [22] | 王文佳.小檗碱的抗奶牛乳房炎机制研究[D].武汉:华中农业大学,2022. |
| WANG W J.Mechanism of berberine against bovine mastitis[D].Wuhan:Huazhong Agricultural University,2022.(in Chinese) | |
| [23] | ARMAN K,ERGÜN S,TEMIZ E,et al.The interrelationship between HER2 and Casp3/8 with apoptosis in different cancer cell lines[J].Mol Biol Rep,2014,41(12):8031-8036 |
| [24] | LV Z,ZHANG J,PING J,et al.Salvianolic acid B inhibits ERK and p38 MAPK signaling in TGF-β1-stimulated human hepatic stellate cell line (LX-2) via distinct pathways[J].Evid Based Complement Alternat Med,2012:960128. |
| [25] | 梁瀞云,唐 燕,司马玲,等.基于MAPK信号通路的荔枝核总黄酮对肝纤维化大鼠作用机制的研究 [J].中药材,2023,46 (11):2856-2860. |
| LIANG J Y,TANG Y,SIMA L,et al.Study on the mechanism of total flavonoids from Litchi chinensis seeds on hepatic fibrosis in rats based on MAPK signaling pathway[J].Journal of Chinese Medicinal Materials,2023,46(11):2856-2860.(in Chinese) | |
| [26] | 裴德翠,熊传银,毕爱芬,等.小檗碱通过调节凋亡相关基因的表达抑制金黄色葡萄球菌诱导的ECV-304细胞凋亡[J].辽宁中医杂志,2015,42(1):35-37. |
| PEI D C,XIONG C Y,BI A F,et al.Berberine inhibits Staphylococcus aureus-induced apoptosis of ECV-304 cells by regulating the expression of apoptosis-related genes[J].Liaoning Journal of Traditional Chinese Medicine,2015,42(1):35-37.(in Chinese) | |
| [27] | MEIXNER A,KARRETH F,KENNER L,et al.Jun and JunD-dependent functions in cell proliferation and stress response[J].Cell Death Differ,2010,17(9):1409-19. |
| [28] | 朱铁梁,张 莉,齐 刚,等.绞股蓝总皂苷对全脑缺血再灌注大鼠海马区c-Jun蛋白表达的抑制作用[J].武警医学,2007(5):347-351. |
| ZHU T L,ZHANG L,QI G,et al.Inhibitory effect of gypenosides on c-Jun protein expression in hippocampus of rats with global cerebral ischemia-reperfusion[J].Medical Journal of the Chinese People’s Armed Police Forces,2007(5):347-351.(in Chinese) | |
| [29] | 张子昂,董 倩,官瑞丽,等.盐酸小檗碱通过抑制TNF-α/caspase-8/caspase-3信号通路减轻急性低氧暴露引起的肝损伤[J].细胞与分子免疫学杂志,2022,38(1):48-53. |
| ZHANG Z A,DONG Q,GUAN R L,et al.Berberine hydrochloride alleviates acute hypoxic liver injury by inhibiting TNF-α/caspase-8/caspase-3 signaling pathway[J].Chinese Journal of Cellular and Molecular Immunology,2022,38(1):48-53.(in Chinese) | |
| [30] | MUKAI M,KUSAMA T,HAMANAKA Y,et al.Cross talk between apoptosis and invasion signaling in cancer cells through caspase-3 activation[J].Cancer Res,2005,65(20):9121-9125. |
| [31] | 吴晓艳,李珏宏,李昌平,等.小檗碱对非酒精性脂肪性肝病大鼠的作用[J].广东医学,2015,36(10):1492-1496. |
| WU X Y,LI J H,LI C P,et al.Effect of berberine on non-alcoholic fatty liver disease in rats[J].Guangdong Medical Journal,2015,36(10):1492-1496.(in Chinese) |
| [1] | DING Tao, MIAO Yuhang, XIN Jie, MA Wenyan, FU Huailin, HU Jumei, DU Jun. Analysis of the Anti-inflammatory Mechanism of Portulaca oleracea L. on Mastitis in Dairy Cows Based on Network Pharmacology and Molecular Docking Technology [J]. Acta Veterinaria et Zootechnica Sinica, 2026, 57(1): 500-512. |
| [2] | YANG Shubo, YUAN Qingxin, CHEN Qibai, WANG Pei, GAO Dongyang, LI He, SONG Jun. Preparation and Intracellular Antibacterial Activity of Liposomes Encapsulating Staphylococcus aureus Bacteriophage [J]. Acta Veterinaria et Zootechnica Sinica, 2025, 56(9): 4638-4645. |
| [3] | ZHAO Yujie, WAN Baoxia, WANG Jiaqi, SUN Siyu, LENG Xinyang, CUI Yizhe. Mechanism of Action of Dandelion Addition to Ration to Enhance the Immune Performance of Geese Based on Network Pharmacological Analysis [J]. Acta Veterinaria et Zootechnica Sinica, 2025, 56(9): 4698-4707. |
| [4] | LI Xiaodie, PAN Shiqin, WANG Lu, CHENG Zhentao, OU Deyuan, SONG Xuqin, YANG Jian. Research Progress on the Anti-inflammatory Mechanism of Traditional Chinese Veterinary Medicine based on Network Pharmacology [J]. Acta Veterinaria et Zootechnica Sinica, 2025, 56(8): 3701-3721. |
| [5] | LIU Yuze, YU Zhuoya, GONG Zhiguo, REN Peipei, ZHAO Jiamin, MAO Wei, ZHANG Shuangyi, FENG Shuang. The Impact of Lipoprotein on the Secretion of Inflammatory Mediators and the Synthesis of Prostaglandin E2 in Bovine Bone Marrow-derived Macrophages Infected with Staphylococcus aureus [J]. Acta Veterinaria et Zootechnica Sinica, 2025, 56(7): 3474-3483. |
| [6] | ZHANG Manqi, ZHAO Bingyu, WEN Ruru, ZHANG Jingwen, SUN Mengran, ZHAN Leyang, GOU Jingxuan, SONG Xiangjun. Prokaryotic Expression of the T6SS Effector Protein Tse1 and Its inhibitory Effect on Staphylococcus aureus [J]. Acta Veterinaria et Zootechnica Sinica, 2025, 56(6): 2917-2926. |
| [7] | CHEN Zehan, ZHANG Ruoyi, LIN Huiying, ZENG Chunli, LIN Fu, LI Jian. Anti-inflammatory Effects of Chelidonium majus on IPEC-J2 Cells based on HPLC Fingerprint and Network Pharmacology [J]. Acta Veterinaria et Zootechnica Sinica, 2025, 56(5): 2466-2480. |
| [8] | JI Xing, LI Jun, WANG Ran, HE Tao. Research Progress on Virulence Regulation and Antivirulence Drugs of Staphylococcus aureus [J]. Acta Veterinaria et Zootechnica Sinica, 2025, 56(4): 1594-1607. |
| [9] | FENG Haipeng, ZHANG Kang, ZHANG Jingyan, LU Xiaorong, LI Jianxi. The Potential Drugs Screening against IBV Infection based on Network Pharmacology and Molecular Docking and Validation in vitro [J]. Acta Veterinaria et Zootechnica Sinica, 2025, 56(12): 6397-6410. |
| [10] | NIE Lianhua, FAN Wenyan, LI Mengya, DING Chunhai, WU Zihao, WANG Zhihao, LI Fangfang, JIANG Wei, HAN Xiangan, WANG Haidong. Analysis of Virulence and Antibiotic Resistance Genes in 78 Strains of Staphylococcus aureus from Chicken Arthritis Sources [J]. Acta Veterinaria et Zootechnica Sinica, 2025, 56(11): 5670-5682. |
| [11] | ZHANG Beiwen, LI Hongxi, WENG Chengzhen, HUANG Xinxin, LI Xiaobing, QIU Longxin, CHEN Hongbo. The Mechanism of Bidens pilosa L. in the Treatment of Bacterial Diarrhea in Poultry based on Network Pharmacological Analysis and Experimental Verification [J]. Acta Veterinaria et Zootechnica Sinica, 2025, 56(11): 5683-5696. |
| [12] | Hengjie CUI, Jinlong QIN, Zhihao ZHU, Xue BAO, Shaowen LI, Xianrong MENG. Correlation Analysis of Benzalkonium Bromide Sensitivity and Biofilm Formation Ability in Staphylococcus aureus [J]. Acta Veterinaria et Zootechnica Sinica, 2024, 55(8): 3669-3677. |
| [13] | Anlin WEN, Yunyun YANG, Yongrong LUO, Ying YANG, Zhentao CHENG, Deyuan OU, Ming WEN. Network Pharmacologic Analysis and Experimential Verification on the Mechanism of Coptidis Rhizoma in Preventing Duck Viral Enteritis [J]. Acta Veterinaria et Zootechnica Sinica, 2024, 55(7): 3225-3233. |
| [14] | HE Xiaolan, ZHAO Yankun, MENG Lu, LIU Huimin, GAO Jiaojiao, ZHENG Nan. Research Progress in Heteroresistance of Staphylococcus aureus [J]. Acta Veterinaria et Zootechnica Sinica, 2024, 55(4): 1432-1445. |
| [15] | XU Junjie, ZHANG Lutong, WANG Jinjie, CHEN Xiaochen, HE Weixian, CAI Chuanjiang, CHU Guiyan, YANG Gongshe. Exploring the Effect of Epimedium on Estrus of Gilts Based on Multiomics and Network Pharmacology [J]. Acta Veterinaria et Zootechnica Sinica, 2024, 55(4): 1615-1628. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||