





畜牧兽医学报 ›› 2025, Vol. 56 ›› Issue (11): 5335-5351.doi: 10.11843/j.issn.0366-6964.2025.11.001
岳怡冰1,2( ), 李俊良1(
), 李俊良1( ), 包斌武1,3, 高晨1, 陈燕1, 朱波1, 张路培1, 王泽昭1, 高会江1, 高雪1,*(
), 包斌武1,3, 高晨1, 陈燕1, 朱波1, 张路培1, 王泽昭1, 高会江1, 高雪1,*( ), 黄永震2,*(
), 黄永震2,*( ), 李俊雅1,*(
), 李俊雅1,*( )
)
收稿日期:2025-04-16
出版日期:2025-11-23
发布日期:2025-11-27
通讯作者:
高雪,黄永震,李俊雅
E-mail:yueyibing2023@163.com;lijunliang@caas.cn;gaoxue@caas.cn;hyzsci@nwafu.edu.cn;lijunya@caas.cn
作者简介:岳怡冰(2001-),女,河南洛阳人,硕士生,主要从事动物遗传育种与繁殖研究,E-mail: yueyibing2023@163.com岳怡冰和李俊良为同等贡献作者
基金资助:
YUE Yibing1,2( ), LI Junliang1(
), LI Junliang1( ), BAO Binwu1,3, GAO Chen1, CHEN Yan1, ZHU Bo1, ZHANG Lupei1, WANG Zezhao1, GAO Huijiang1, GAO Xue1,*(
), BAO Binwu1,3, GAO Chen1, CHEN Yan1, ZHU Bo1, ZHANG Lupei1, WANG Zezhao1, GAO Huijiang1, GAO Xue1,*( ), HUANG Yongzhen2,*(
), HUANG Yongzhen2,*( ), LI Junya1,*(
), LI Junya1,*( )
)
Received:2025-04-16
Online:2025-11-23
Published:2025-11-27
Contact:
GAO Xue, HUANG Yongzhen, LI Junya
E-mail:yueyibing2023@163.com;lijunliang@caas.cn;gaoxue@caas.cn;hyzsci@nwafu.edu.cn;lijunya@caas.cn
摘要:
OMEGA编辑系统被认为是CRISPR-Cas系统的祖先,因其较小的尺寸和RNA引导的DNA切割能力成为基因编辑工具研究的热点。本文详细介绍了OMEGA编辑系统的基本结构和功能,并综述了通过对OMEGA系统核酸酶进行优化设计和引导RNA工程化改造等策略来提高其编辑效率和特异性;或通过融合脱氨酶实现碱基编辑,拓展其应用范围,为新工具的开发和优化提供参考。
中图分类号:
岳怡冰, 李俊良, 包斌武, 高晨, 陈燕, 朱波, 张路培, 王泽昭, 高会江, 高雪, 黄永震, 李俊雅. OMEGA基因编辑系统:结构、功能及其优化方案的研究进展[J]. 畜牧兽医学报, 2025, 56(11): 5335-5351.
YUE Yibing, LI Junliang, BAO Binwu, GAO Chen, CHEN Yan, ZHU Bo, ZHANG Lupei, WANG Zezhao, GAO Huijiang, GAO Xue, HUANG Yongzhen, LI Junya. Research Progress on OMEGA Gene Editing System: Structure, Function, and Optimization Strategies[J]. Acta Veterinaria et Zootechnica Sinica, 2025, 56(11): 5335-5351.

图 1
IscB蛋白结构与IscB-ωRNA-DNA三元结构图[17] a. IscB蛋白结构域示意图,主要包含N端PLMP结构域、RuvC结构域、HNH结构域、WED结构域和TI结构域。PLMP结构域位于蛋白的N端,参与蛋白的定位;RuvC域和HNH域与DNA切割相关;WED域主要与蛋白和DNA的结合相关;TI结构域与蛋白的稳定性相关。b. IscB-ωRNA-DNA三元结构图。ωRNA通过与DNA的互补序列结合,指导蛋白识别特定的DNA序列;WED结构域通过其特定氨基酸残基与DNA的单链末端形成氢键;RuvC结构域通过其催化活性位点与DNA的主链形成直接的化学键,参与DNA的切割;TI为TAM相互作用区;TS为目标链;NTS为非目标链"
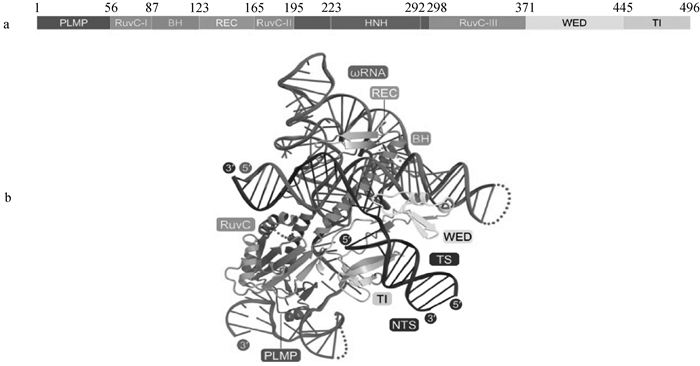
图 2
IsrB蛋白结构与IsrB-ωRNA-DNA三元结构图[23] a. IsrB蛋白结构域示意图,主要包含N端PLMP结构域、RuvC结构域、WED结构域、TI结构域;PLMP结构域位于蛋白的N端参与蛋白的定位;RuvC域与DNA切割相关;WED域主要与蛋白和DNA的结合相关;TI结构域与蛋白的稳定性相关。b. IsrB-ωRNA-DNA三元结构图,ωRNA通过与DNA的互补序列结合,指导蛋白识别特定的DNA序列;WED结构域通过其特定氨基酸残基与DNA的单链末端形成氢键;RuvC结构域通过其催化活性位点与DNA的主链形成直接的化学键,参与DNA的切割;PLL为磷酸锁环;TI为TAM相互作用区;TS为目标链;NTS为非目标链"
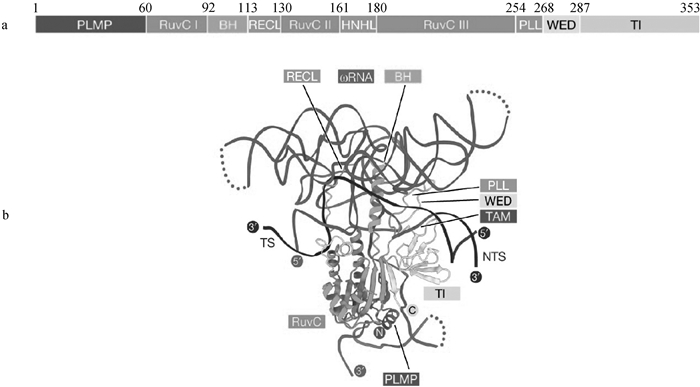
图 3
TnpB蛋白结构与TnpB-reRNA-DNA三元结构图[32] a. TnpB蛋白结构域示意图,包含N端识别(Rec)和C端核酸酶(Nuc)叶,Rec叶包括REC结构域和楔形(WED),Nuc叶包括RuvC结构域和ZnF结构域;REC域主要与蛋白识别DNA相关;WED域主要与蛋白和DNA的结合相关;RuvC域与DNA切割相关;ZnF域与DNA结合相关。b. TnpB-reRNA-DNA三元结构图,reRNA通过与DNA的互补序列结合,指导蛋白识别特定的DNA序列;WED结构域通过其特定氨基酸残基与DNA的单链末端形成氢键;RuvC结构域通过其催化活性位点与DNA的主链形成直接的化学键,参与DNA的切割;ZnF结构域通过其锌指结构与DNA的特定序列结合,使结合更加稳定;TS为目标链;NTS为非目标链"
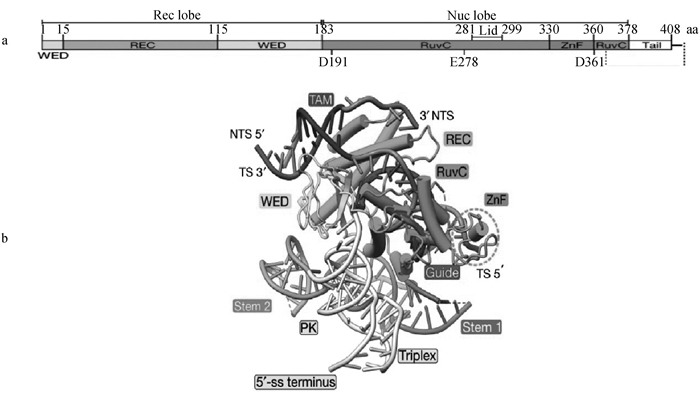
表 1
OMEGA编辑系统的优化策略及进展"
| 时间 Date | 优化系统 Optimization system | 蛋白大小/aa Protein size | ωRNA长度/nt ωRNA length | 编辑对象 Editing target | 优化方案 Optimization strategies | 优化后编辑效率 Optimized editing efficiency | 优化后碱基编辑器编辑效率 Optimized base editor efficiency | 参考文献 Reference | ||
| OMEGA系统核酸酶设计 The OMEGA system nuclease design | ωRNA工程化改造 ωRNA engineering | 融合脱氨酶 Fusion deaminase for base editing | ||||||||
| 2023/5/25 | OgeuIscB-ωRNA系统 | 496 | 222 | HEK293T细胞 | 1.氨基酸替换:E85R、H369R、S387R和S457R得到IscB*;2.融合T5核酸外切酶(T5E):基于enIscB将T5E融合到IscB蛋白C端得到enIscB-T5E | R1茎环截短15 bp(M3)、R2茎环第10碱基对被G-C碱基对独立替换,得到ωRNA* | miABE和miCBE | enIscB-T5E(60.61%±27.43%)在23个目标位点显示出与SpG Cas9(53.25%±30.92%)相当的编辑效率 | miABE编辑效率可达52.37%~60.06%;miCBE编辑效率可达50.17±2.49%~66.42%±1.13%。 | [ |
| 2024/7/8 | OgeuIscB-ωRNA系统 | 496 | 222 | HEK293T、A549 and HeLa细胞 | 氨基酸替换:M102R、F137K、V159K、N281R、Q324R、Y327K、H368R和L393K得到enOgeuIscB | 将茎环中的无序区域使用更稳定的四环“GAAA”取代,并恢复茎环2中的错配,得到ωRNA-v2;在ωRNA-v2的基础上恢复茎环1中的错配得到ωRNA-v13 | enOgeuIscB BE | 在6个基因组位点上观察到64.5%~87.3%的编辑效率 | 优化后的编辑效率达到36.1%±21.1%,野生型OgeuIscB-ωRNA的编辑率为20.3%±21.2% | [ |
| 2024/7/8 | OgeuIscB-ωRNA系统 | 496 | 222 | HEK293T细胞 | 氨基酸替换:D97K、F138N、S431K、S457R得到IscBnQM | 删除了ωRNA的部分茎环1和3′末端生成了优化的ωRNA变体 | SIminiBEs | 与野生型IscBn相比,IscBnQM编辑效率从3.16%~10.13%提高到70.85%~84.60%,优化的ωRNA变体比野生型编辑效率提高了约2倍 | 编辑效率可达50%~60%,提高约2~3倍 | [ |
| 2024/8/15 | IscB.m16系统 | 400左右 | 200~300 | HEK293T细胞 | 氨基酸替换:E326R、P460S、T462H和T459E得到IscB.m16RESH | R1茎环截短13 bp、R5茎环截短10 bp、第24、25、57、79、117和189 bp的碱基对替换为G-C,将其命名为enωRNA | IscB.m16*-ABE和IscB.m16*-CBE | 工程化的IscB.m16*编辑效率在多个位点都超过了40%,比野生型提高2~9倍 | IscB.m16*-ABE(46.15%±4.08%)显示出显著高于IscB.m16-ABE(9.19%± 2.34%);IscB.m16*-CBE编辑效率显著高于SpG-CBE | [ |
| 2024/8/22 | OgeuIscB-ωRNA系统 | 496 | 222 | HEK293T细胞 | 1.氨基酸替换:D96R、E84R、V159R,融合HMG-D结构域;得到IscB-DIscB-D2.核定位信号优化:进行了SV40 NLS融合,优化后的变体被命名为eIscB-D | P1茎环截短15 bp、P2茎环截短4 bp、P5茎环截短4 bp、末端发夹截断、在第11位用G-C配对替换A-U配对和在第103位用A替换C以形成A-U配对,命名为eωRNA | eiABE,eiCBE | eIscB-D编辑效率最高可达91.3%;与原始IscB相比,工程化的eIscB-D/eωRNA系统的活性平均增加了20.2倍 | eiABE效率高达58.3%,eiCBE效率为35.7%~79.2%(平均61.9%) | [ |
| 2024/11/26 | enIscB系统 | 496 | 163 | HEK293T细胞 | 无 | 茎环2的完全去除、茎环3中88号位置的A: U配对替换以及3′尾序列的14个核苷酸缺失,最终长度为163 nt,命名为ωRNA*-v2 | 对miABE、miCBE进行AAV包装,在酪氨酸血症小鼠模型中实现了疾病纠正 | 编辑效率可达87.3% | 碱基编辑效率可达62.2% | [ |
| 2024/1/27 | ISDra2 TnpB系统 | 408 | 231 | HEK293T、小鼠N2a细胞、植物细胞(nicotiana benthamiana,本氏烟草) | 无 | S1、S2和S3的同时缺失并用5′-GAAA-3′环序列替换SL3亚结构域的序列得到ωRNA*变体(99nt) | 无 | 与野生型ωRNA相比,ωRNA*的基因编辑效率提高了两倍(70%左右),与SaCas9效率相当 | 无 | [ |
| 2024/3/19 | ISDra2 TnpB系统 | 408 | 231 | HEK293T和HeLa细胞,小鼠NIH/3T3细胞 | 蛋白结构域截短:截短CTD结构域,TnpB379 | 无 | 无 | TnpB379(29.0%±12.3%)编辑效率高于TnpB(26.4%±10.7%) | 无 | [ |
| 2024/6/28 | ISDra2 TnpB系统 | 408 | 231 | 水稻、拟南芥 | 1.密码子优化:将ISDra2 TnpB基因的密码子替换为植物细胞偏好的密码子;2.启动子优化:使用Pol-II启动子(ZmUbi)替代Pol-III(RNA聚合酶Ⅲ)启动子(OsU3)得到TnpB2 | 无 | D191A突变,得到TnpB-ABE | 在植物基因组中实现了平均高达33.58% 的编辑效率 | 编辑效率只有0.42%~1.12% | [ |
| 2024/8/7 | TnpB系统 | 408 | 231 | 水稻、拟南芥、青蒿、丹参、黄芩、靛蓝和党参 | 1.核定位信号优化:TnpB载体的两个末端融合了真核核定位信号,将TnpB与黄色荧光蛋白(YFP)融合生成了YFP-TnpB构建体;2.启动子优化:由U6和UBQ1启动子驱动reRNA和TnpB载体 | 无 | 无 | 编辑效率最高达30% | 无 | [ |
| 2024/8/21 | IsDge10 TnpB系统 | 400左右 | 200~300 | 水稻 | 启动子优化:IsDge10蛋白在ZmUbi1启动子下表达,7个sgRNA在OsUbi启动子下表达并融合了HH-HDV双核酶系统 | 无 | 无 | 在水稻原生质体中的效率为4.3% 至18.2% | 无 | [ |
| 2024/9/23 | ISDra2 TnpB系统 | 408 | 231 | HEK293T细胞、小鼠肝脏、大脑 | 1.氨基酸替换:蛋白第76位引入氨基酸替换(K76A、K76C和K76S);2.核定位信号优化:ARC13设计(在C端包含额外的GS接头和二分NLS序列);3.密码子优化:对TnpBmax系统进行了哺乳动物密码子优化 | 利用TEEP将原始的ωRNA支架缩短至117个核苷酸,在ωRNA的3′端融合HDV核酶得到mini-ωRNA | TnpBmax系统的RuvC结构域中引入了突变(D191A)并融合了TadA8e16生成碱基编辑器 | 编辑率比野生型提高了1.3倍;在小鼠肝脏中实现了高达75.3%的编辑效率,在小鼠脑中实现了高达65.9%的编辑效率 | 编辑效率可达16.6% | [ |
| 2025/2/16 | ISDra2 TnpB系统 | 408 | 231 | HEK293T;本氏烟草 | 1.氨基酸替换:P282I单点突变使效率提升四倍;深度突变扫描(DMS)筛选到6个组合突变的变体(eTnpB1a-eTnpB1f),eTnpB1e效率最高;2.蛋白结构域截短:C端截短至376 aa,是最小活性TnpB截断变体 | 茎2区域的“铰链”区域(rA-37-rU-44)发生的碱基缺失和替换编辑效率最高 | 无 | eTnpB1e在PDS1-1位点编辑效率为33%,与WT ISDra2 TnpB (< 1%)相比增加了50倍以上 | 无 | [ |
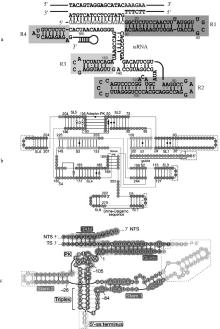
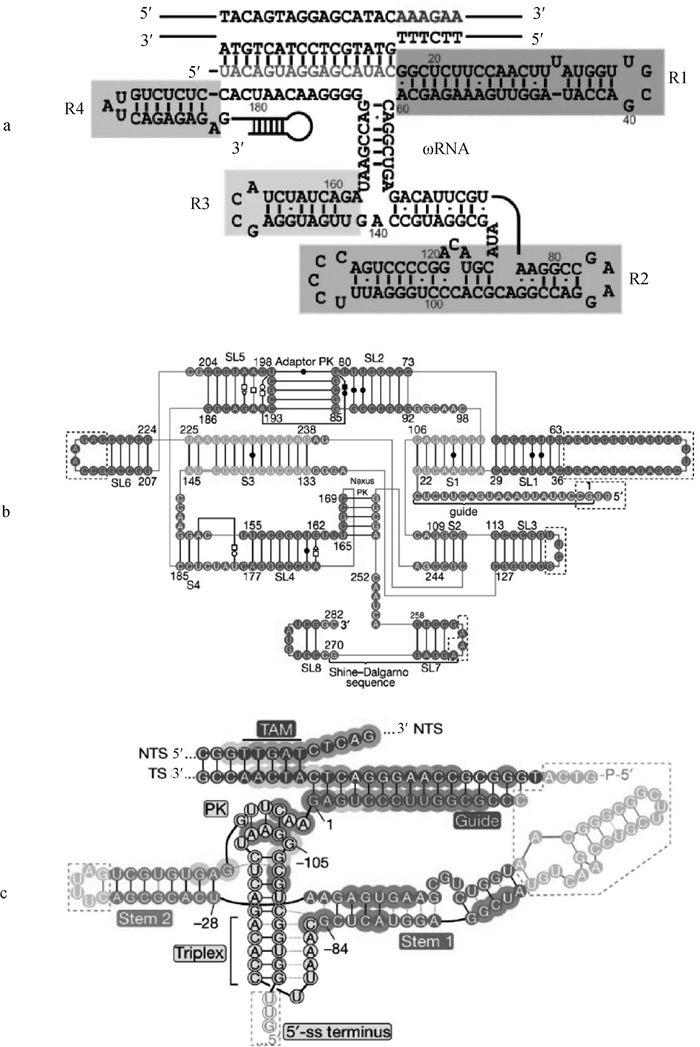
图 4
IscB、IsrB、TnpB系统引导RNA的预测二级结构[23, 32, 36] a. IscB系统的ωRNA分为4个区域(R1、R2、R3和R4);b. IsrB系统的ωRNA分为4个茎(S1-4)和8个茎环(SL1-8);adaptor PK是SL2和SL5形成的接头假结结构;nexus PK是SL4、S2和SL7之间的区域形成的纽带假结结构;Shine-Dalgarno序列是细菌中的一种核糖体结合位点,位于mRNA的起始密码子(通常是AUG)上游约8个碱基的位置;c. TnpB系统的reRNA分为stem1和2;Triplex为三螺旋结构;Stem为茎环结构;5′-ss terminus为5′端终止序列;PK为假结结构;TS为目标链;NTS为非目标链;TAM为目标相邻基序"
| 1 |
GALLAGHERD N,HABERJ E.Repair of a site-specific DNA cleavage: Old-school lessons for Cas9-mediated gene editing[J].ACS Chem Biol,2018,13(2):397-405.
doi: 10.1021/acschembio.7b00760 |
| 2 |
SCHERERS,DAVISR W.Replacement of chromosome segments with altered DNA sequences constructed in vitro[J].Proc Natl Acad Sci U S A,1979,76(10):4951-4955.
doi: 10.1073/pnas.76.10.4951 |
| 3 |
DOYONY,VOT D,MENDELM C,et al.Enhancing zinc-finger-nuclease activity with improved obligate heterodimeric architectures[J].Nat Methods,2011,8(1):74-79.
doi: 10.1038/nmeth.1539 |
| 4 |
王玮玮,刘瑞琪,吴勇延,等.CRISPR/Cas9基因编辑系统研究进展及其在动物基因编辑研究中的应用[J].畜牧兽医学报,2016,47(7):1299-1305.
doi: 10.11843/j.issn.0366-6964.2016.07.001 |
|
WANGW W,LIUR Q,WUY Y,et al.The Progress of CRISPR/Cas9 System and Its Application in Animal Genetic Engineering[J].Acta Veterinaria et Zootechnica Sinica,2016,47(7):1299-1305.
doi: 10.11843/j.issn.0366-6964.2016.07.001 |
|
| 5 |
CUIX,JID,FISHERD A,et al.Targeted integration in rat and mouse embryos with zinc-finger nucleases[J]. Nat Biotechnol,2011,29(1):64-67.
doi: 10.1038/nbt.1731 |
| 6 |
MILLERJ C,TANS,QIAOG,et al.A TALE nuclease architecture for efficient genome editing[J].Nat Biotechnol,2011,29(2):143-148.
doi: 10.1038/nbt.1755 |
| 7 |
CERMAKT,DOYLEE L,CHRISTIANM,et al.Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting[J].Nucleic Acids Res,2011,39(12):e82.
doi: 10.1093/nar/gkr218 |
| 8 |
HOCKEMEYERD,WANGH,KIANIS,et al.Genetic engineering of human pluripotent cells using TALE nucleases[J].Nat Biotechnol,2011,29(8):731-734.
doi: 10.1038/nbt.1927 |
| 9 | ZHANGF,VOYTASD F.Targeted mutagenesis in Arabidopsis using zinc-finger nucleases[J].Methods Mol Biol,2011,701,167-177. |
| 10 |
WHYTEJ J,ZHAOJ,WELLSK D,et al.Gene targeting with zinc finger nucleases to produce cloned eGFP knockout pigs[J].Mol Reprod Dev,2011,78(1):2.
doi: 10.1002/mrd.21271 |
| 11 |
WIEDENHEFTB,STERNBERGS H,DOUDNAJ A.RNA-guided genetic silencing systems in bacteria and archaea[J]. Nature,2012,482(7385):331-338.
doi: 10.1038/nature10886 |
| 12 |
CONGL,RANF A,COXD,et al.Multiplex genome engineering using CRISPR/Cas systems[J].Science,2013,339(6121):819-823.
doi: 10.1126/science.1231143 |
| 13 |
ALTAE-TRANH,KANNANS,DEMIRCIOGLUF E,et al.The widespread IS200/IS605 transposon family encodes diverse programmable RNA-guided endonucleases[J].Science,2021,374(6563):57-65.
doi: 10.1126/science.abj6856 |
| 14 |
KANNANS,ALTAE-TRANH,ZHUS,et al.Evolution-guided protein design of IscB for persistent epigenome editing in vivo[J].Nat Biotechnol,2025,
doi: 10.1038/s41587-025-02655-3 |
| 15 |
WANGF,MAS,ZHANGS,et al.CRISPR beyond: harnessing compact RNA-guided endonucleases for enhanced genome editing[J].Sci China Life Sci,2024,67(12):2563-2574.
doi: 10.1007/s11427-023-2566-8 |
| 16 |
KONGX,LIT,YANGH.AAV-mediated gene therapies by miniature gene editing tools[J].Sci China Life Sci,2024,67(12):2540-2553.
doi: 10.1007/s11427-023-2608-5 |
| 17 |
KATOK,OKAZAKIS,KANNANS,et al.Structure of the IscB-ωRNA ribonucleoprotein complex, the likely ancestor of CRISPR-Cas9[J]. Nat Commun,2022,13(1):6719.
doi: 10.1038/s41467-022-34378-3 |
| 18 | XUC,NIUX,SUNH,et al.Conversion of IscB and Cas9 into RNA-guided RNA editors[J].Cell,2025,S0092-8674(25):00854-2. |
| 19 | KAPITONOVV V,MAKAROVAK S,KOONINE V.ISC, a novel group of bacterial and archaeal DNA transposons that encode Cas9 homologs[J].J Bacteriol,2015,198(5):797-807. |
| 20 |
WANGK,WANGJ,YANGX,et al.Structural insights into Type Ⅱ-D Cas9 and its robust cleavage activity[J].Nat Commun,2025,16(1):7396.
doi: 10.1038/s41467-025-62128-8 |
| 21 |
GAOJ,WANGH,SUNJ,et al.Highly efficient genome editing in Bacillus subtilis via miniature DNA nucleases IscB[J]. Synth Syst Biotechnol,2025,10(4):1215-1223.
doi: 10.1016/j.synbio.2025.06.012 |
| 22 |
LVJ,JINJ,DINGL,et al.Directed evolution of ogeuiscb with enhanced activity in human cells[J].FASEB J,2025,39(8):e70570.
doi: 10.1096/fj.202500082R |
| 23 |
HIRANOS,KAPPELK,ALTAE-TRANH,et al.Structure of the OMEGA nickase IsrB in complex with ωRNA and target DNA[J].Nature,2022,610(7932):575-581.
doi: 10.1038/s41586-022-05324-6 |
| 24 |
YUZ,SHEQ.Genome editing from Cas9 to IscB: Backwards and forwards towards new breakthroughs[J].Eng Microbiol,2021,1,100004.
doi: 10.1016/j.engmic.2021.100004 |
| 25 |
MARQUARTK F,MATHISN,MOLLAYSAA,et al.Effective genome editing with an enhanced ISDra2 TnpB system and deep learning-predicted ωRNAs[J].Nat Methods,2024,21(11):2084-2093.
doi: 10.1038/s41592-024-02418-z |
| 26 |
JIANGK,LIMJ,SGRIZZIS,et al.Programmable RNA-guided endonucleases are widespread in eukaryotes and their viruses[J]. bioRxiv[Preprint],2023,
doi: 10.1101/2023.06.13.544871 |
| 27 |
KARVELIST,DRUTEIKAG,BIGELYTEG,et al.Transposon-associated TnpB is a programmable RNA-guided DNA endonuclease[J].Nature,2021,599(7886):692-696.
doi: 10.1038/s41586-021-04058-1 |
| 28 |
FUW,MAJ,WANGZ,et al.Mechanisms and engineering of a miniature type V-N CRISPR-Cas12 effector enzyme[J].Nat Commun,2025,16(1):5667.
doi: 10.1038/s41467-025-61290-3 |
| 29 |
SAJJADM W,NAQVIR Z,AMINI.Plant genome editing goes viral[J].Trends Biotechnol,2025,43(7):1520-1522.
doi: 10.1016/j.tibtech.2025.05.005 |
| 30 |
WEIY,GAOP,PAND,et al.Engineering eukaryotic transposon-encoded Fanzor2 system for genome editing in mammals[J]. Nat Chem Biol,2025,
doi: 10.1038/s41589-025-01902-7 |
| 31 |
JIANGK,GOOTENBERGJ S,ABUDAYYEHO O.Fanzors, a family of eukaryotic RNA-guided DNA endonucleases[J].FEBS Lett,2025,599(8):1089-1093.
doi: 10.1002/1873-3468.70038 |
| 32 |
SASNAUSKASG,TAMULAITIENEG,DRUTEIKAG,et al.TnpB structure reveals minimal functional core of Cas12 nuclease family[J].Nature,2023,616(7956):384-389.
doi: 10.1038/s41586-023-05826-x |
| 33 | KLEINSTIVERB P,SOUSAA A,WALTONR T,et al.Engineered CRISPR-Cas12a variants with increased activities and improved targeting ranges for gene, epigenetic and base editing[J].Nat Biotechnol,2020,38(7):901. |
| 34 | ZHANGH,KONGX,XUEM,et al.An engineered xCas12i with high activity, high specificity, and broad PAM range[J].Protein Cell,2023,14(7):538-543. |
| 35 |
CHATTERJEEP,JAKIMON,LEEJ,et al.An engineered ScCas9 with broad PAM range and high specificity and activity[J].Nat Biotechnol,2020,38(10):1154-1158.
doi: 10.1038/s41587-020-0517-0 |
| 36 |
HAND,XIAOQ,WANGY,et al.Development of miniature base editors using engineered IscB nickase[J].Nat Methods,2023,20(7):1029-1036.
doi: 10.1038/s41592-023-01898-9 |
| 37 |
XUEN,HONGD,ZHANGD,et al.Engineering IscB to develop highly efficient miniature editing tools in mammalian cells and embryos[J]. Mol Cell,2024,84(16):3128-3140.
doi: 10.1016/j.molcel.2024.07.007 |
| 38 |
STOTTK,WATSONM,BOSTOCKM J,et al.Structural insights into the mechanism of negative regulation of single-box high mobility group proteins by the acidic tail domain[J]. J Biol Chem,2014,289(43):29817-29826.
doi: 10.1074/jbc.M114.591115 |
| 39 |
KLASSJ,MURPHYFV 4TH,FOUTSS,et al.The role of intercalating residues in chromosomal high-mobility-group protein DNA binding, bending and specificity[J].Nucleic Acids Res,2003,31(11):2852-2864.
doi: 10.1093/nar/gkg389 |
| 40 |
YINS,ZHANGM,LIUY,et al.Engineering of efficiency-enhanced Cas9 and base editors with improved gene therapy efficacies[J].Mol Ther,2023,31(3):744-759.
doi: 10.1016/j.ymthe.2022.11.014 |
| 41 |
YANH,TANX,ZOUS,et al.Assessing and engineering the IscB-ωRNA system for programmed genome editing[J].Nat Chem Biol,2024,20(12):1617-1628.
doi: 10.1038/s41589-024-01669-3 |
| 42 |
XIAOQ,LIG,HAND,et al.Engineered IscB-ωRNA system with expanded target range for base editing[J].Nat Chem Biol,2025,21(1):100-108.
doi: 10.1038/s41589-024-01706-1 |
| 43 | THORNTON B W, WEISSMAN R F, TRAN R V, et al. Latent activity in TnpB revealed by mutational scanning[J]. bioRxiv[Preprint], 2025, 637750. |
| 44 |
KIMD Y,CHUNGY,LEEY,et al.Hypercompact adenine base editors based on transposase B guided by engineered RNA[J].Nat Chem Biol,2022,18(9):1005-1013.
doi: 10.1038/s41589-022-01077-5 |
| 45 |
CHENW,MAJ,WUZ,et al.Cas12n nucleases, early evolutionary intermediates of type V CRISPR, comprise a distinct family of miniature genome editors[J].Mol Cell,2023,83(15):2768-2780.
doi: 10.1016/j.molcel.2023.06.014 |
| 46 |
ALTAE-TRANH,SHMAKOVS A,MAKAROVAK S,et al.Diversity, evolution, and classification of the RNA-guided nucleases TnpB and Cas12[J].Proc Natl Acad Sci U S A,2023,120(48):e2308224120.
doi: 10.1073/pnas.2308224120 |
| 47 |
WANGM,SUNZ,LIUY,et al.Hypercompact TnpB and truncated TnpB systems enable efficient genome editing in vitro and in vivo[J].Cell Discov,2024,10(1):31.
doi: 10.1038/s41421-023-00645-w |
| 48 |
WUY,YUANQ,ZHUY,et al.Improving FnCas12a genome editing by exonuclease fusion[J].CRISPR J,2020,3(6):503-511.
doi: 10.1089/crispr.2020.0073 |
| 49 |
YINJ,LUR,XINC,et al.Cas9 exo-endonuclease eliminates chromosomal translocations during genome editing[J].Nat Commun,2022,13(1):1204.
doi: 10.1038/s41467-022-28900-w |
| 50 |
WANGW,LIS,YANGJ,et al.Exploiting the efficient Exo: Cas12i3-5M fusions for robust single and multiplex gene editing in rice[J].J Integr Plant Biol,2025,67(5):1246-1253.
doi: 10.1111/jipb.13850 |
| 51 |
WANGW,YANL,LIJ,et al.Engineering a robust Cas12i3 variant-mediated wheat genome editing system[J].Plant Biotechnol J,2025,23(3):860-873.
doi: 10.1111/pbi.14544 |
| 52 |
LIAOK,CHENK,MAS,et al.exoCasMINI: A T5 exonuclease fused CRISPR-Cas12f system with enhanced gene editing efficiency[J].iScience,2025,28(8):113171.
doi: 10.1016/j.isci.2025.113171 |
| 53 |
MAS,LIAOK,CHENK,et al.hpCasMINI: An engineered hypercompact CRISPR-Cas12f system with boosted gene editing activity[J].Nat Commun,2025,16(1):5001.
doi: 10.1038/s41467-025-60124-6 |
| 54 | REZAEI S, MONCADA-RESTREPO M, LENG S, et al. Synthesizing unmodified, supercoiled circular DNA molecules in vitro[J]. bioRxiv[Preprint], 2025, 01.24.634800. |
| 55 |
SHUIS,WANGS,LIUJ.Systematic investigation of the effects of multiple SV40 nuclear localization signal fusion on the genome editing activity of purified SpCas9[J]. Bioengineering (Basel),2022,9(2):83.
doi: 10.3390/bioengineering9020083 |
| 56 |
NⅡNUMAS,WAKEY,NAKAGAWAY,et al.Importance of nuclear localization signal-fused Cas9 in the production of genome-edited mice via embryo electroporation[J].Biochem Biophys Res Commun,2023,685,149140.
doi: 10.1016/j.bbrc.2023.149140 |
| 57 |
SALIMI-JEDAA,ESGHAEIM,HOSSEINKEYVANI,et al.Inhibition of HIV-1 replication using the CRISPR/cas9-no NLS system as a prophylactic strategy[J].Heliyon,2022,8(9):e10483.
doi: 10.1016/j.heliyon.2022.e10483 |
| 58 |
LVZ,CHENW,FANGS,et al.Targeted mutagenesis in Arabidopsis and medicinal plants using transposon-associated TnpB[J].J Integr Plant Biol,2024,66(10):2083-2086.
doi: 10.1111/jipb.13758 |
| 59 |
WANGL,HANH.Strategies for improving the genome-editing efficiency of class 2 CRISPR/Cas system[J].Heliyon,2024,10(19):e38588.
doi: 10.1016/j.heliyon.2024.e38588 |
| 60 |
NALLEYM J,BANERJEES,HUANGM Y,et al.Near 100% efficient homology-dependent genome engineering in the human fungal pathogen Cryptococcus neoformans[J].G3 (Bethesda),2025,15(8):jkaf118.
doi: 10.1093/g3journal/jkaf118 |
| 61 |
WALLACEK A,GERSTENBERGT L,ENNISC L,et al.A differentiated β-globin gene replacement strategy uses heterologous introns to restore physiological expression[J].Mol Ther,2025,33(4):1407-1419.
doi: 10.1016/j.ymthe.2025.02.036 |
| 62 |
KARMAKARS,PANDAD,PANDAS,et al.A miniature alternative to Cas9 and Cas12: Transposon-associated TnpB mediates targeted genome editing in plants[J].Plant Biotechnol J,2024,22(10):2950-2953.
doi: 10.1111/pbi.14416 |
| 63 |
ZHANGY,RENQ,TANGX,et al.Expanding the scope of plant genome engineering with Cas12a orthologs and highly multiplexable editing systems[J].Nat Commun,2021,12(1):1944.
doi: 10.1038/s41467-021-22330-w |
| 64 |
ZHAOY,BOEKEJ D.CRISPR-Cas12a system in fission yeast for multiplex genomic editing and CRISPR interference[J].Nucleic Acids Res,2020,48(10):5788-5798.
doi: 10.1093/nar/gkaa329 |
| 65 |
ZHONGZ,LIUS,LIUX,et al.Intron-based single transcript unit CRISPR systems for plant genome editing[J].Rice (N Y),2020,13(1):8.
doi: 10.1186/s12284-020-0369-8 |
| 66 |
ZHANGR,TANGX,HEY,et al.IsDge10 is a hypercompact TnpB nuclease that confers efficient genome editing in rice[J].Plant Commun,2024,5(11):101068.
doi: 10.1016/j.xplc.2024.101068 |
| 67 |
JINEKM,CHYLINSKIK,FONFARAI,et al.A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity[J].Science,2012,337(6096):816-821.
doi: 10.1126/science.1225829 |
| 68 |
GUOR,SUNX,WANGF,et al.Engineered IscB-ωRNA system with improved base editing efficiency for disease correction via single AAV delivery in mice[J].Cell Rep,2024,43(11):114973.
doi: 10.1016/j.celrep.2024.114973 |
| 69 |
HANL,HUY,MOQ,et al.Engineering miniature IscB nickase for robust base editing with broad targeting range[J].Nat Chem Biol,2024,20(12):1629-1639.
doi: 10.1038/s41589-024-01670-w |
| 70 |
LIZ,GUOR,SUNX,et al.Engineering a transposon-associated TnpB-ωRNA system for efficient gene editing and phenotypic correction of a tyrosinaemia mouse model[J]. Nat Commun,2024,15(1):831.
doi: 10.1038/s41467-024-45197-z |
| 71 |
RICHTERM F,ZHAOK T,ETONE,et al.Phage-assisted evolution of an adenine base editor with improved Cas domain compatibility and activity[J].Nat Biotechnol,2020,38(7):883-891.
doi: 10.1038/s41587-020-0453-z |
| 72 |
ARANTESP R,CHENX,SINHAS,et al.Dimerization of the deaminase domain and locking interactions with Cas9 boost base editing efficiency in ABE8e[J].Nucleic Acids Res,2024,52(22):13931-13944.
doi: 10.1093/nar/gkae1066 |
| 73 |
包斌武,邹惠影,李俊良,等.基因编辑技术的研究进展[J].畜牧兽医学报,2025,56(1):1-14.
doi: 10.11843/j.issn.0366-6964.2025.01.001 |
|
BAOB W,ZOUH Y,LIJ L,et al.Research progress in gene editing technology[J].Acta Veterinaria et Zootechnica Sinica,2025,56(1):1-14.
doi: 10.11843/j.issn.0366-6964.2025.01.001 |
|
| 74 |
WANGY,PROSENDE,MEIL,et al.A novel strategy to engineer DNA polymerases for enhanced processivity and improved performance in vitro[J].Nucleic Acids Res,2004,32(3):1197-1207.
doi: 10.1093/nar/gkh271 |
| [1] | 邵嘉皓, 张艳婕, 赵永聚. N6-甲基腺苷(m6A)修饰在畜禽遗传育种与繁殖中的调控作用研究进展[J]. 畜牧兽医学报, 2025, 56(10): 4774-4786. |
| [2] | 曹雨, 周铂涵, 许琦, 袁子翱, 苏蕊, 吕琦, 李金泉, 张燕军, 王瑞军, 王志英. 整合eQTL和GWAS数据识别潜在功能基因位点在动物遗传育种中的研究进展[J]. 畜牧兽医学报, 2025, 56(10): 4759-4773. |
| [3] | 骞里, 梁忙, 邓天宇, 杜丽丽, 李柯安宁, 邱诗元, 薛青青, 张路培, 高雪, 徐凌洋, 郑彩宏, 李俊雅, 高会江. 基于自编码器整合转录组数据提升基因组预测的准确性[J]. 畜牧兽医学报, 2025, 56(9): 4410-4421. |
| [4] | 李佳鹏, 刘庆, 孙佳钰, 马泽芳, 崔凯. 基于转录组和蛋白质组分析筛选银黑狐毛色形成的关键基因[J]. 畜牧兽医学报, 2025, 56(9): 4379-4392. |
| [5] | 郑云畅, 侯睿霖, 梁晓贺, 杨利丹, 张银蛟, 霍浩楠, 陈玮娜, 张萃, 李世杰. 牛FOXP2基因的单等位基因表达和DNA甲基化状态分析[J]. 畜牧兽医学报, 2025, 56(9): 4369-4378. |
| [6] | 王彦博, 张笑梦, 景秀娟, 冯肖艺, 张元庆, 赵学明. 纳米粒子在动物种质资源冷冻保存的研究进展[J]. 畜牧兽医学报, 2025, 56(9): 4156-4164. |
| [7] | 张洋, 王仲发, 李旻娟, 何玉楠, 关伟军. 治疗运动性损伤肌腱来源间充质干细胞体外培养与鉴定[J]. 畜牧兽医学报, 2025, 56(8): 3813-3825. |
| [8] | 张帆, 曾威, 周傲. 畜禽基因编辑抗病育种研究进展[J]. 畜牧兽医学报, 2025, 56(7): 3047-3056. |
| [9] | 周锐, 吴德, 车炼强, 林燕, 冯斌, 方正锋. N6-腺苷甲基化调控脂肪生成的研究进展[J]. 畜牧兽医学报, 2025, 56(5): 1995-2003. |
| [10] | 包斌武, 邹惠影, 李俊良, 高晨, 高会江, 杜振伟, 张博玉, 李俊雅, 高雪. 基因编辑技术的研究进展[J]. 畜牧兽医学报, 2025, 56(1): 1-14. |
| [11] | 闵祥玉, 卫佳丽, 许彪, 刘汇涛, 郑军军, 王桂武. 梅花鹿鹿茸全长转录组测序及鹿茸产量相关基因的挖掘[J]. 畜牧兽医学报, 2024, 55(12): 5549-5566. |
| [12] | 贾宏霞, 刘在霞, 周乐, 鲍艳春, 霍晨曦, 左鹏鹏, 谷明娟, 娜日苏, 张文广. 基因组选择在肉牛中的研究进展[J]. 畜牧兽医学报, 2024, 55(9): 3757-3768. |
| [13] | 夏振涛, 王楠, 王婉洁, 周期律, 黄雷, 牟玉莲. pAPN基因敲除的IPEC-J2介导的TGEV感染特征分析[J]. 畜牧兽医学报, 2024, 55(8): 3395-3407. |
| [14] | 张肖旭, 李昊, 冯平捷, 杨豪, 李新月, 吕冉, 潘章源, 储明星. 单细胞转录组测序技术在家养动物中的应用[J]. 畜牧兽医学报, 2024, 55(8): 3276-3287. |
| [15] | 刘雯雯, 董发明, 毕延震. 多基因编辑技术的发展及其在畜牧种质创新中的应用[J]. 畜牧兽医学报, 2024, 55(8): 3267-3275. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
