





Acta Veterinaria et Zootechnica Sinica ›› 2025, Vol. 56 ›› Issue (4): 1755-1767.doi: 10.11843/j.issn.0366-6964.2025.04.024
• Animal Biotechnology and Reproduction • Previous Articles Next Articles
ZHANG Hongyan1( ), WANG Shanpeng1, CAO Hailiang2, MIN Lingjiang1, ZHOU Kaifeng3,*(
), WANG Shanpeng1, CAO Hailiang2, MIN Lingjiang1, ZHOU Kaifeng3,*( ), ZHU Zhendong1,*(
), ZHU Zhendong1,*( )
)
Received:2024-09-13
Online:2025-04-23
Published:2025-04-28
Contact:
ZHOU Kaifeng, ZHU Zhendong
E-mail:13356221095@163.com;zkf2050@163.com;ZZD2020@qau.edu.cn
CLC Number:
ZHANG Hongyan, WANG Shanpeng, CAO Hailiang, MIN Lingjiang, ZHOU Kaifeng, ZHU Zhendong. Study on Freeze Resistance and Fatty Acid Composition of Boar Sperm[J]. Acta Veterinaria et Zootechnica Sinica, 2025, 56(4): 1755-1767.
Table 1
Sperm motility before freezing in semen with differential freeze-thaw tolerance"
| 参数 Parameter | GFE | PFE |
| 活率/% TM | 93.80±0.24 | 90.65±2.33 |
| 活力/% PM | 39.64±1.42 | 38.30±2.67 |
| 曲线速度/(μm·s-1 )VCL | 124.90±4.62 | 129.45±3.77 |
| 直线速度/(μm·s-1 )VSL | 34.39±0.74a | 32.72±1.59b |
| 平均路径速度/(μm·s-1 )VAP | 62.83±2.51 | 61.14±1.98 |
| 鞭打频率/Hz BCF | 39.18±2.14 | 40.67±1.54 |
| 头部侧向位移幅度/μm ALH | 7.53±0.76 | 7.02±0.42 |
| 直线指数/% STR | 59.98±0.81 | 56.07±1.92 |
| 线性指数/% LIN | 30.76±0.51 | 28.77±0.48 |
| 振动指数/% WOB | 47.69±0.64 | 50.30±0.71 |
Table 2
Sperm motility after thawing of semen with differential freeze-thaw tolerance"
| 参数 Parameter | GFE | PFE |
| 活率/% TM | 63.00±0.15a | 34.91±2.33b |
| 活力/% PM | 29.14±2.51a | 13.90±1.94b |
| 曲线速度/(μm·s-1 )VCL | 104.97±8.70a | 95.45±2.89b |
| 直线速度/(μm·s-1 )VSL | 35.35±2.12a | 30.22±1.47b |
| 平均路径速度/(μm·s-1 )VAP | 52.72±3.67 | 46.21±1.76 |
| 鞭打频率/Hz BCF | 43.19±1.82 | 39.06±1.11 |
| 头部侧向位移幅度/μm ALH | 7.42±0.35 | 6.88±0.12 |
| 直线指数/% STR | 62.54±0.22 | 63.56±0.19 |
| 线性指数/% LIN | 32.40±0.64 | 32.53±0.36 |
| 振动指数/% WOB | 49.78±0.49 | 49.42±0.35 |

Fig. 1
Detection of acrosome and membrane integrity in semen with differential freezing tolerance before freezing A. Flow cytometry is used to detect the integrity of sperm acrosome and membrane: Q1 represents sperm with damaged acrosome and intact membrane, Q2 represents sperm with damaged acrosome and membrane, Q3 represents sperm with intact acrosome and damaged membrane, and Q4 represents sperm with intact acrosome and intact membrane; B. Statistics of sperm acrosome integrity before freezing; C. Statistics of sperm membrane integrity before freezing. Different letters in the figure indicate significant differences (P < 0.05), while the same or no letter indicate no significant differences (P>0.05). The following pictures are the same"
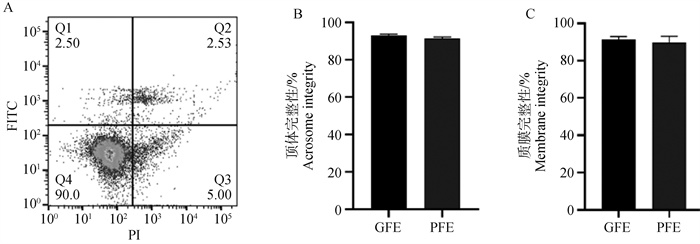

Fig. 2
Detection of acrosome and membrane integrity in semen with differential freeze-thaw tolerance after freezing and thawing A. Flow cytometry is used to detect the integrity of sperm acrosome and membrane: Q1 represents sperm with damaged acrosome and intact membrane, Q2 represents sperm with damaged acrosome and membrane, Q3 represents sperm with intact acrosome and damaged membrane, and Q4 represents sperm with intact acrosome and intact membrane; B. Statistics of sperm acrosome integrity after freezing and thawing; C. Statistics of sperm membrane integrity after freezing and thawing"


Fig. 4
Detection of high membrane fluidity levels in semen with differential freeze-thaw resistance before freezing A. Flow cytometry is used to detect the fluidity level of sperm membrane before freezing: Q2 represents sperm with damaged cell membrane, Q3 represents sperm with intact cell membrane and membrane fluidity, P1 represents sperm with intact cell membrane and low membrane fluidity, and P2 represents sperm with intact cell membrane and high membrane fluidity; B. Statistics of high membrane fluidity of sperm before freezing"
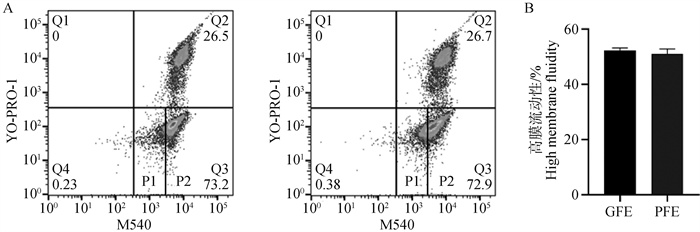

Fig. 5
Detection of high membrane fluidity levels in thawed semen with differential freeze-thaw resistance A. Flow cytometry was used to detect the level of sperm membrane fluidity after freezing and thawing: Q2 represents sperm with damaged cell membranes, Q3 represents sperm with intact cell membranes and membrane fluidity, P1 represents sperm with intact cell membranes and low membrane fluidity, and P2 represents sperm with intact cell membranes and high membrane fluidity; B. Statistics of high membrane fluidity of sperm after freezing and thawing"
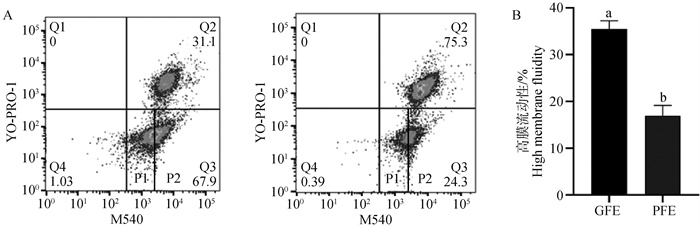
Table 4
Sperm motility after freezing-thawing of semen mixed with lipid"
| 处理 Treatment | 0%LM | 0.01%LM | 0.1%LM | 1%LM |
| 活率/% TM | 38.27±1.52b | 41.28±1.17b | 51.53±1.46a | 37.16±1.93c |
| 活力/% PM | 20.46±0.88b | 22.19±1.42b | 33.74±0.92a | 20.53±1.45c |
| 曲线速度/(μm·s-1 )VCL | 111.14±4.51a | 103.76±2.32b | 114.50±3.18a | 102.82±3.72b |
| 直线速度/(μm·s-1 )VSL | 40.16±1.73b | 39.71±2.01b | 44.32±0.87a | 36.31±1.24c |
| 平均路径速度/(μm·s-1 )VAP | 56.45±1.33a | 56.65±1.61a | 58.69±1.28a | 51.27±1.57b |
| 鞭打频率/Hz BCF | 44.61±1.16b | 45.17±0.96b | 47.21±1.42a | 45.29±1.17b |
| 头部侧向位移幅度/μm LH | 6.79±0.56ab | 6.45±0.85b | 7.36±0.15a | 6.07±0.74b |
| 直线指数/% STR | 67.12±2.32 | 67.55±1.52 | 68.24±1.64 | 68.92±2.37 |
| 线性指数/% LIN | 37.70±1.14ab | 38.69±0.76a | 38.15±1.21a | 34.80±0.67b |
| 振动指数/% WOB | 52.38±1.62 | 50.14±1.37 | 51.97±1.42 | 49.82±1.52 |
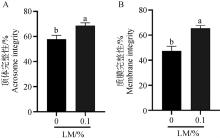
Fig. 8
Detection of acrosome and membrane integrity after freezing and thawing of semen with lipid mixture A. Statistical analysis of sperm acrosome integrity after freezing and thawing of semen with 0.1% LM; B. Statistical analysis of sperm membrane integrity after freezing and thawing of semen with 0.1% LM"
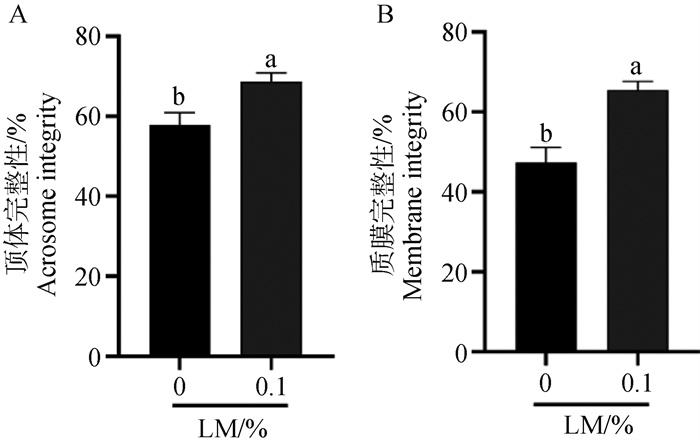
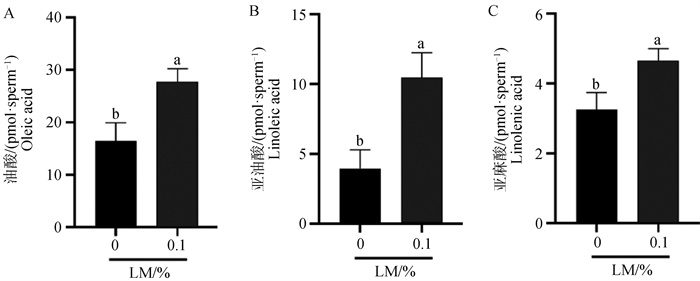
Fig. 11
Detection of differential fatty acid content after freezing and thawing of semen with lipid mixture A. Statistical analysis of oleic acid content after freezing and thawing of semen with 0.1% LM; B. Statistical analysis of linoleic acid content after freezing and thawing of semen with 0.1% LM; C. Statistical analysis of linolenic acid content after freezing and thawing of semen with 0.1% LM"
| 1 | 管旺东, 杨文辉, 张静. 冷冻精液对母猪繁殖性能的影响[J]. 现代畜牧科技, 2024, 109 (6): 25- 27. |
| GUAN W D , YANG W H , ZHANG J . The effect of frozen semen on the reproductive performance of sows[J]. Technical Advisor for Animal Husbandry, 2024, 109 (6): 25- 27. | |
| 2 | 任忠伟, 苏智辉, 杜烨青, 等. 牛精液冷冻保存技术研究进展[J]. 畜牧兽医杂志, 2020, 39 (2): 50- 53. |
| REN Z W , SU Z H , DU Y Q , et al. Research progress on bovine cryopreservation[J]. Journal of Animal Science and Veterinary Medicine, 2020, 39 (2): 50- 53. | |
| 3 |
SPINACI M , PERTEGHELLA S , CHLAPANIDAS T , et al. Storage of sexed boar spermatozoa: limits and perspectives[J]. Theriogenology, 2016, 85 (1): 65- 73.
doi: 10.1016/j.theriogenology.2015.05.018 |
| 4 |
YANEZ-ORTIZ I , CATALAN J , RODRIGUEZ-GIL J E , et al. Advances in sperm cryopreservation in farm animals: cattle, horse, boar and sheep[J]. Anim Reprod Sci, 2022, 246, 106904.
doi: 10.1016/j.anireprosci.2021.106904 |
| 5 | YESTE M . Recent advances in boar sperm cryopreservation: State of the art and current perspectives[J]. Reprod Domest Anim, 2015, 50 (Suppl 2): 71- 79. |
| 6 | SRIDHARAN T B , VICKRAM A S . Evolving trends in cryopreservation and parameters influencing semen extender preparation-a prospective review[J]. Cryo Letters, 2016, 37 (3): 196- 205. |
| 7 |
OKAZAKI T , SHIMADA M . New strategies of boar sperm cryopreservation: development of novel freezing and thawing methods with a focus on the roles of seminal plasma[J]. Anim Sci J, 2012, 83 (9): 623- 629.
doi: 10.1111/j.1740-0929.2012.01034.x |
| 8 |
SINGH M , MOLLIER R T , PATTON R N , et al. Linseed oil in boar's diet improved in vivo fertility and antioxidant status[J]. Reprod Domest Anim, 2023, 58 (1): 27- 38.
doi: 10.1111/rda.14249 |
| 9 |
HAMMERSTEDT R H , GRAHAM J K , NOLAN J P . Cryopreservation of mammalian sperm: what we ask them to survive[J]. J Androl, 1990, 11 (1): 73- 88.
doi: 10.1002/j.1939-4640.1990.tb01583.x |
| 10 |
PARKS J E , LYNCH D V . Lipid composition and thermotropic phase behavior of boar, bull, stallion, and rooster sperm membranes[J]. Cryobiology, 1992, 29 (2): 255- 266.
doi: 10.1016/0011-2240(92)90024-V |
| 11 |
WHITE I G . Lipids and calcium uptake of sperm in relation to cold shock and preservation: a review[J]. Reprod Fertil Dev, 1993, 5 (6): 639- 658.
doi: 10.1071/RD9930639 |
| 12 | ZHU Z , LI R , FAN X , et al. Resveratrol improves boar sperm quality via 5'AMP-activated protein kinase activation during cryopreservation[J]. Oxid Med Cell Longev, 2019, 9 (4): 219- 226. |
| 13 | ZHANG W , LI Y , ZHU Z . Carboxylated epsilon-poly-L-lysine supplementation of the freezing extender improves the post-thawing boar sperm quality[J]. Animals (Basel), 2022, 12 (13): 6- 12. |
| 14 | ZHU Z , ZHANG W , LI R , et al. Reducing the glucose level in pre-treatment solution improves post-thaw boar sperm quality[J]. Front Vet Sci, 2022, 5 (30): 9- 15. |
| 15 |
CASAS I , ALTHOUSE G C . The protective effect of a 17 degrees C holding time on boar sperm plasma membrane fluidity after exposure to 5 degrees C[J]. Cryobiology, 2013, 66 (1): 69- 75.
doi: 10.1016/j.cryobiol.2012.11.006 |
| 16 |
WILLIAMSON P , MATTOCKS K , SCHLEGEL R A . Merocyanine 540, a fluorescent probe sensitive to lipid packing[J]. Biochim Biophys Acta, 1983, 732 (2): 387- 393.
doi: 10.1016/0005-2736(83)90055-X |
| 17 | HOVING L R , HEIJINK M , VAN HARMELEN V , et al. GC-MS analysis of medium-and long-chain fatty acids in blood samples[J]. Methods Mol Biol, 2018, 1730, 257- 265. |
| 18 | BECCARIA M , FRANCHINA F A , NASIR M , et al. Investigation of mycobacteria fatty acid profile using different ionization energies in GC-MS[J]. Anal Bioanal Chem, 2018, 410 (30): 7987- 7996. |
| 19 | 张宇霆. 猪精子差异耐冻性机制与耐冻性生物标志物筛选[D]. 哈尔滨: 东北农业大学, 2021. |
| ZHANG Y T. Mechanism of Porcine Sperm Differential freezability and identification of the sperm freezability biomarkers[D]. Harbin: Northeast Agricultural University, 2021. (in Chinese) | |
| 20 | 杜学海. 猪冷冻精液生产与应用研究进展[J]. 养猪, 2024 (1): 21- 24. |
| DU X H . Research progress on production and application of frozen boar semen[J]. Swine Production, 2024 (1): 21- 24. | |
| 21 | SANTIAGO-MORENO J , TOLEDANO-DIAZ A , CASTANO C , et al. Review: Sperm cryopreservation in wild small ruminants: morphometric, endocrine and molecular basis of cryoresistance[J]. Animal, 2023, 17 (Suppl 1): 100741. |
| 22 | ZANG S , YANG X , YE J , et al. Quantitative phosphoproteomics explain cryopreservation-induced reductions in ram sperm motility[J]. J Proteomics, 2024, 298, 105153. |
| 23 | MAZUR P , LEIBO S P , CHU E H . A two-factor hypothesis of freezing injury. Evidence from Chinese hamster tissue-culture cells[J]. Exp Cell Res, 1972, 71 (2): 345- 355. |
| 24 | JOHNSON L A , WEITZE K F , FISER P , et al. Storage of boar semen[J]. Anim Reprod Sci, 2000, 62 (1-3): 143- 172. |
| 25 | DI NISIO A , DE TONI L , SABOVIC I , et al. Lipidomic profile of human sperm membrane identifies a clustering of lipids associated with semen quality and function[J]. Int J Mol Sci, 2023, 25 (1): 297. |
| 26 | CONTRERAS M J , ARIAS M E , FUENTES F , et al. Cellular and molecular consequences of stallion sperm cryopreservation: recent approaches to improve sperm survival[J]. J Equine Vet Sci, 2023, 126, 104499. |
| 27 | BARGUREN M , LOPEZ D J , ESCRIBA P V . The effect of natural and synthetic fatty acids on membrane structure, microdomain organization, cellular functions and human health[J]. Biochim Biophys Acta, 2014, 1838 (6): 1518- 1528. |
| 28 | MURANUSHI N , TAKAGI N , MURANISHI S , et al. Effect of fatty acids and monoglycerides on permeability of lipid bilayer[J]. Chem Phys Lipids, 1981, 28 (3): 269- 279. |
| 29 | MAZUR P . Equilibrium, quasi-equilibrium, and nonequilibrium freezing of mammalian embryos[J]. Cell Biophys, 1990, 17 (1): 53- 92. |
| 30 | LI Z , FI J JIANG K , et al. Hyperbaric oxygen improves cognitive impairment induced by hypoxia via upregulating the expression of oleic acid and MBOAT2 of mice[J]. Antioxidants (Basel), 2024, 13 (11): 1320. |
| 31 | NIKOLOPOULOU M , SOUCEK D A , VARY J C . Modulation of the lipid composition of boar sperm plasma membranes during an acrosome reaction in vitro[J]. Arch Biochem Biophys, 1986, 250 (1): 30- 37. |
| 32 | AURICH C , ORTEGA FERRUSOLA C , PENA VEGA F J , et al. Seasonal changes in the sperm fatty acid composition of Shetland pony stallions[J]. Theriogenology, 2018, 107, 149- 153. |
| 33 | CASTELLANO C A , AUDET I , LAFOREST J P , et al. Fish oil diets alter the phospholipid balance, fatty acid composition, and steroid hormone concentrations in testes of adult boars[J]. Theriogenology, 2011, 76 (6): 1134- 1145. |
| 34 | 白小龙, 吴德, 林燕, 等. 添加不同植物油和L-肉碱对公猪精液品质和性欲的影响[J]. 动物营养学报, 2011, 23 (8): 1361- 1369. |
| BAI X L , WU D , LIN Y , et al. Different vegetable oils and L-carnitine influence libido and semen characteristics of breeding boars[J]. Chinese Journal of Animal Nutrition, 2011, 23 (8): 1361- 1369. | |
| 35 | 袁崇善, 王军, 吕文发. 多不饱和脂肪酸对动物精液品质影响的研究进展[J]. 中国畜牧兽医, 2022, 49 (4): 1422- 1429. |
| YUAN C S , WANG J , LV W F . Research progress on effects of polyunsaturated fatty acids on animal semen quality[J]. Chinese animal husbandry and veterinary medicine, 2022, 49 (4): 1422- 1429. | |
| 36 | ZHU Z , LI R , FENG C , et al. Exogenous oleic acid and palmitic acid improve boar sperm motility via enhancing mitochondrial beta-oxidation for ATP generation[J]. Animals (Basel), 2020, 10 (4): 591- 606. |
| 37 | COORAY A , KIM J H , CHAE M R , et al. Perspectives on potential fatty acid modulations of motility associated human sperm ion channels[J]. Int J Mol Sci, 2022, 23 (7): 3718. |
| 38 | LI Y , HU Y , WANG Z , et al. IKBA phosphorylation governs human sperm motility through ACC-mediated fatty acid beta-oxidation[J]. Commun Biol, 2023, 6 (1): 323. |
| 39 | WATERHOUSE K E , HOFMO P O , TVERDAL A , et al. Within and between breed differences in freezing tolerance and plasma membrane fatty acid composition of boar sperm[J]. Reproduction, 2006, 131 (5): 887- 894. |
| 40 | VARMA S , MOLANGIRI A , KONA S R , et al. Fetal exposure to endocrine disrupting-bisphenol A (BPA) alters testicular fatty acid metabolism in the adult offspring: relevance to sperm maturation and quality[J]. Int J Mol Sci, 2023, 24 (4): 3769. |
| 41 | COLLODEL G , CASTELLINI C , LEE J C , et al. Relevance of fatty acids to sperm maturation and quality[J]. Oxid Med Cell Longev, 2020, 5, 7038124. |
| [1] | MA Yingtian, JIANG Luyao, LI Zengkai, QIN Jianping, ZHAO Jianhua, HE Yufang, SONG Yuxuan, ZHANG Lei. Effect of Cyanidin-3-rutinoside on Cryopreservation of Semen of Dairy Sheep [J]. Acta Veterinaria et Zootechnica Sinica, 2025, 56(4): 1768-1778. |
| [2] | LIANG Entang, LI Huaxuan, CHEN Shuaicheng, LI Guo, SUN Gege, ZAN Linsen. Effect of Genistein on Semen Cryopreservation of Bull [J]. Acta Veterinaria et Zootechnica Sinica, 2025, 56(2): 700-710. |
| [3] | Jianhua DONG, Xiaoyi FENG, Baigao YANG, Chongyang LI, Hongmei PAN, Lihua LÜ, Xueming ZHAO. Advances in Cryopreservation of Porcine Embryo [J]. Acta Veterinaria et Zootechnica Sinica, 2024, 55(11): 4796-4807. |
| [4] | FENG Xiaoyi, XU Xi, ZHANG Hang, YANG Baigao, ZHANG Peipei, HAO Haisheng, DU Weihua, ZHU Huabin, CUI Kai, ZHAO Xueming. Advances in Cryopreservation of Bovine Embryo in vitro [J]. Acta Veterinaria et Zootechnica Sinica, 2023, 54(2): 451-462. |
| [5] | CHEN Siying, SUN Yawen, LI Kang, LIU Shuo, HAO Haisheng, DU Weihua, ZOU Huiying, ZHU Huabin, PANG Yunwei. Application of Microfluidic Technologies in Livestock in vitro Embryo Production [J]. Acta Veterinaria et Zootechnica Sinica, 2023, 54(12): 4889-4897. |
| [6] | LI Chunyan, ZHANG Yan, Lü Chunrong, DENG Weidong, QUAN Guobo. Research Progress on Antioxidant Mechanisms of Melatonin and Its Application in Cryopreservation of Mammalian Spermatozoa [J]. Acta Veterinaria et Zootechnica Sinica, 2023, 54(11): 4468-4476. |
| [7] | CAI Shaoli, XU Jiehuan, HE Mengqian, ZHANG Defu, SUN Lingwei, ZHANG Shushan, LI Wanjun, WU Caifeng, ZHU Xing, DAI Jianjun. Effects of Forskolin on Lipid Degradation and Cryopreservation of Porcine Oocytes [J]. Acta Veterinaria et Zootechnica Sinica, 2023, 54(1): 178-188. |
| [8] | WANG Meng, YANG Chaoqun, WU Silin, TAN Jianbing, DU Xinze, LI Zhenxing, ZAN Linsen, YANG Wucai. Impact of Lycopene on Semen Cryopreservation and Fresh Semen Quality of Qinchuan Bull [J]. Acta Veterinaria et Zootechnica Sinica, 2022, 53(12): 4507-4517. |
| [9] | WANG Junyue, DAI Jianjun, ZHANG Shushan, SUN Lingwei, WU Caifeng, ZHANG Defu. Effects of High Pressure Homogeneous Egg Yolk on Apoptosis of Boar Sperm Cryopreservation [J]. Acta Veterinaria et Zootechnica Sinica, 2021, 52(8): 2190-2199. |
| [10] | WANG Qi, WANG Changjian, WEI Zongyou, LU Hanxi, YAO Xiaolei, YANG Hua, WANG Feng, ZHANG Yanli. Study on the Role of AMPK Activator in Cryopreservation of Sheep Semen [J]. Acta Veterinaria et Zootechnica Sinica, 2020, 51(12): 3033-3045. |
| [11] | LI Rui-lan, ZHANG Tong, LIU Zhi-hong, WANG Rui-jun, LI Jin-quan, ZHANG Jia-xin. The Effect of Glucose on Cryopreservation and Metabolism of Cashmere Goat Sperm [J]. ACTA VETERINARIA ET ZOOTECHNICA SINICA, 2018, 49(9): 2054-2062. |
| [12] | LI Yu-hua, FU Jie-li, LI Pei-fei, YANG Qiang-zhen, WANG Li-rui, XIE Wei-yi, LI Xin-hong. Effects of Staphylococcus aureus on Motility and Protein Phosphorylation Modification of Boar Sperm during Storage in vitro [J]. ACTA VETERINARIA ET ZOOTECHNICA SINICA, 2017, 48(9): 1665-1673. |
| [13] | LIN Jie,WANG Xu-rong,WANG Lei,ZHANG Jing-yan,WANG Xue-zhi,MENG Jia-ren,YANG Zhi-qiang,LI Jian-xi. Cryopreservation of the Mammary Gland and Improvement on the Culture of the Primary Epithelial Cells in Holstein Dairy Cows [J]. ACTA VETERINARIA ET ZOOTECHNICA SINICA, 2016, 47(5): 1067-1074. |
| [14] | WANG Xin,DAI Jian-jun,WU Cai-feng,ZHANG Shu-shan,WU Yun-long,ZHANG De-fu. Preliminary Studies on Apoptosis and Apoptotic Pathways of Frozen Boar Spermatozoa [J]. ACTA VETERINARIA ET ZOOTECHNICA SINICA, 2015, 46(8): 1341-1347. |
| [15] | LIU Yang,HUANG He-lu,DONG Hai-tao,JIN Yi. Effect of LDL from Pigeon Egg Yolk on the Expression of HSP70 and Apoptosis-related Gene in Cold Shock and Frozen-thawed Boar Spermatozoa [J]. ACTA VETERINARIA ET ZOOTECHNICA SINICA, 2015, 46(6): 940-948. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||