





Acta Veterinaria et Zootechnica Sinica ›› 2025, Vol. 56 ›› Issue (4): 1843-1853.doi: 10.11843/j.issn.0366-6964.2025.04.032
• Preventive Veterinary Medicine • Previous Articles Next Articles
HUANG Cheng1( ), YANG Zhiyuan1, LIN Jian1, CHENG Huimin1, WANG Mi2, MAO Huilin2, WANG Guoliang3, LIU Guiming3, ZHAO Jicheng1, LIU Yuehuan1,*(
), YANG Zhiyuan1, LIN Jian1, CHENG Huimin1, WANG Mi2, MAO Huilin2, WANG Guoliang3, LIU Guiming3, ZHAO Jicheng1, LIU Yuehuan1,*( )
)
Received:2024-05-30
Online:2025-04-23
Published:2025-04-28
Contact:
LIU Yuehuan
E-mail:huangc_2019@163.com;liuyuehuan@sina.com
CLC Number:
HUANG Cheng, YANG Zhiyuan, LIN Jian, CHENG Huimin, WANG Mi, MAO Huilin, WANG Guoliang, LIU Guiming, ZHAO Jicheng, LIU Yuehuan. Construction and Efficacy Evaluation of mRNA Vaccines against H9 Subtype Avian Influenza Virus[J]. Acta Veterinaria et Zootechnica Sinica, 2025, 56(4): 1843-1853.
Table 2
Primer sequences used for expression determination of cytokine genes in PBMC"
| 基因gene | 引物对序列(5′→3′) (F/R) sequence | 序列号Accession No. |
| IL-4 | AGCACTGCCACAAGAACCTG/TTGCCTGCTGCCGTGGGACAT | NM_001007079.2 |
| IFN-γ | GACAAGTCAAAGCCGCACA/TCAAGTCGTTCATCGGGAGC | NM_205149.2 |
| β-actin | CCCGTGCTGTGTTCCCATCTATCG/GGGTGCTCCTCAGGGGCTACTCTC | NM_205518.2 |

Fig. 2
Recombinant plasmid validation results A. Identification of recombinant plasmid pUC57-HA (M. DL10000 DNA marker; 1. Plasmid pUC57-HA; 2. Double restriction products of plasmid pUC57-HA); B. Identification of recombinant plasmid pUC57-HAe (M. DL10000 DNA marker; 1. Plasmid pUC57-HA; 2. Double restriction products of plasmid pUC57-HA)"

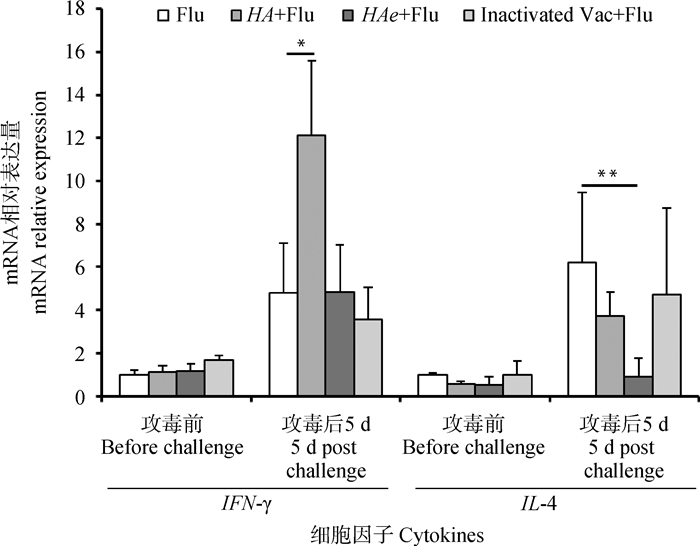
Fig. 7
Transcription of PBMC cytokines before and on the 5th day post viral challenge Flu, HA+Flu, HAe+Flu, and Inactivated Vac+Flu represent challenge control group, HA mRNA LNP group, HAe mRNA LNP group, and inactivated vaccine group, respectively. * represents P < 0.05. ** represents P < 0.01"
| 1 |
GU M , XU L J , WANG X Q , et al. Current situation of H9N2 subtype avian influenza in China[J]. Vet Res, 2017, 48 (1): 49.
doi: 10.1186/s13567-017-0453-2 |
| 2 |
ELADL A H , ARAFAT N , EL-SHAFEI R A , et al. Comparative immune response and pathogenicity of the H9N2 avian influenza virus after administration of Immulant®, based on Echinacea and Nigella sativa, in stressed chickens[J]. Comp Immunol Microbiol Infect Dis, 2019, 65, 165- 175.
doi: 10.1016/j.cimid.2019.05.017 |
| 3 |
WANG J L , CAO Z W , GUO X J , et al. Cytokine expression in three chicken host systems infected with H9N2 influenza viruses with different pathogenicities[J]. Avian Pathol, 2016, 45 (6): 630- 639.
doi: 10.1080/03079457.2016.1193665 |
| 4 |
ALEXANDER D J . Newcastle disease and other avian paramyxoviruses[J]. Rev Sci Tech, 2000, 19 (2): 443- 455.
doi: 10.20506/rst.19.2.1231 |
| 5 |
ABDEL-MONEIM A S , AFIFI M A , EL-KADY M F . Isolation and mutation trend analysis of influenza A virus subtype H9N2 in Egypt[J]. Virol J, 2012, 9, 173.
doi: 10.1186/1743-422X-9-173 |
| 6 |
SIKHT F Z , DUCATEZ M , TOUZANI C D , et al. Avian influenza a H9N2 viruses in Morocco, 2018-2019[J]. Viruses, 2022, 14 (3): 529.
doi: 10.3390/v14030529 |
| 7 |
SUN Y P , PU J , JIANG Z L , et al. Genotypic evolution and antigenic drift of H9N2 influenza viruses in China from 1994 to 2008[J]. Vet Microbiol, 2010, 146 (3-4): 215- 225.
doi: 10.1016/j.vetmic.2010.05.010 |
| 8 |
LIU S , JI K , CHEN J M , et al. Panorama phylogenetic diversity and distribution of type A influenza virus[J]. PLoS One, 2009, 4 (3): e5022.
doi: 10.1371/journal.pone.0005022 |
| 9 |
RAJÃO D S , PÉREZ D R . Universal vaccines and vaccine platforms to protect against influenza viruses in humans and agriculture[J]. Front Microbiol, 2018, 9, 123.
doi: 10.3389/fmicb.2018.00123 |
| 10 |
ABDELWHAB E M , ABDEL-MONEIM A S . Epidemiology, ecology and gene pool of influenza A virus in egypt: will Egypt be the epicentre of the next influenza pandemic?[J]. Virulence, 2015, 6 (1): 6- 18.
doi: 10.4161/21505594.2014.992662 |
| 11 |
KIM Y H , BANG Y J , PARK H J , et al. Inactivated influenza vaccine formulated with single-stranded RNA-based adjuvant confers mucosal immunity and cross-protection against influenza virus infection[J]. Vaccine, 2020, 38 (39): 6141- 6152.
doi: 10.1016/j.vaccine.2020.07.022 |
| 12 |
ELADL A H , FARAG V M , EL-SHAFEI R A , et al. Immunological, biochemical and pathological effects of vitamin C and Arabic gum co-administration on H9N2 avian influenza virus vaccinated and challenged laying Japanese quails[J]. BMC Vet Res, 2022, 18 (1): 408.
doi: 10.1186/s12917-022-03495-y |
| 13 | MEHRABADI M H F , GHALYANCHILANGEROUDI A , GHAFOURI S A , et al. Comparison of autogenous and commercial H9N2 avian influenza vaccines in a challenge with recent dominant virus[J]. Iran J Vet Res, 2020, 21 (2): 109- 114. |
| 14 |
LIU M A . A comparison of plasmid DNA and mRNA as vaccine technologies[J]. Vaccines (Basel), 2019, 7 (2): 37.
doi: 10.3390/vaccines7020037 |
| 15 |
REICHMUTH A M , OBERLI M A , JAKLENEC A , et al. mRNA vaccine delivery using lipid nanoparticles[J]. Ther Deliv, 2016, 7 (5): 319- 334.
doi: 10.4155/tde-2016-0006 |
| 16 | ANDERSON E J. mRNA vaccines and COVID-19-the start of a new era of vaccinology[D]. 2021. |
| 17 |
SCHLAKE T , THESS A , FOTIN-MLECZEK M , et al. Developing mRNA-vaccine technologies[J]. RNA Biol, 2012, 9 (11): 1319- 1330.
doi: 10.4161/rna.22269 |
| 18 |
PARDI N , HOGAN M J , PORTER F W , et al. mRNA vaccines—a new era in vaccinology[J]. Nat Rev Drug Discov, 2018, 17 (4): 261- 279.
doi: 10.1038/nrd.2017.243 |
| 19 |
THESS A , GRUND S , MUI B L , et al. Sequence-engineered mRNA without chemical nucleoside modifications enables an effective protein therapy in large animals[J]. Mol Ther, 2015, 23 (9): 1456- 1464.
doi: 10.1038/mt.2015.103 |
| 20 |
MAUGERI M , NAWAZ M , PAPADIMITRIOU A , et al. Linkage between endosomal escape of LNP-mRNA and loading into EVs for transport to other cells[J]. Nat Commun, 2019, 10 (1): 4333.
doi: 10.1038/s41467-019-12275-6 |
| 21 |
WANG T , WEI F H , LIU J H . Emerging role of mucosal vaccine in preventing infection with avian influenza A viruses[J]. Viruses, 2020, 12 (8): 862.
doi: 10.3390/v12080862 |
| 22 | FAN M L , LIANG B , ZHAO Y Z , et al. Mutations of 127, 183 and 212 residues on the HA globular head affect the antigenicity, replication and pathogenicity of H9N2 avian influenza virus[J]. Transbound Emerg Dis, 2022, 69 (4): e659- e670. |
| 23 | 楚电峰, 于晓璐, 孙化露, 等. mRNA技术在动物疫病疫苗中的研究进展[J]. 中国动物检疫, 2024, 41 (1): 52- 59. |
| CHU D F , YU X L , SUN H L , et al. Research progress on mRNA technology in development of animal vaccines[J]. China Animal Health Inspection, 2024, 41 (1): 52- 59. | |
| 24 |
PETSCH B , SCHNEE M , VOGEL A B , et al. Protective efficacy of in vitro synthesized, specific mRNA vaccines against influenza A virus infection[J]. Nat Biotechnol, 2012, 30 (12): 1210- 1216.
doi: 10.1038/nbt.2436 |
| 25 | 庄忻雨. 流感病毒mRNA疫苗的构建、制备与实验免疫研究[D]. 北京: 军事科学院, 2020. |
| ZHUANG X Y. Construction, preparation and immunogenicity studies of influenza virus mRNA vaccines[D]. Beijing: Academy of Military Science, 2020. | |
| 26 |
XU S K , ZHANG B W , YAO J L , et al. A new H9 influenza virus mRNA vaccine elicits robust protective immunity against infection[J]. Vaccine, 2023, 41 (18): 2905- 2913.
doi: 10.1016/j.vaccine.2023.03.049 |
| 27 |
ZHU J F , PAUL W E . Heterogeneity and plasticity of T helper cells[J]. Cell Res, 2010, 20 (1): 4- 12.
doi: 10.1038/cr.2009.138 |
| 28 |
OKOYE I S , WILSON M S . CD4+ T helper 2 cells-microbial triggers, differentiation requirements and effector functions[J]. Immunology, 2011, 134 (4): 368- 377.
doi: 10.1111/j.1365-2567.2011.03497.x |
| 29 |
DAI M M , XU C G , CHEN W S , et al. Progress on chicken T cell immunity to viruses[J]. Cell Mol Life Sci, 2019, 76, 2779- 2788.
doi: 10.1007/s00018-019-03117-1 |
| [1] | SHI Yuting, HAN Xinyu, ZHONG Muhui, LIN Yaozhong, LIU Tengfei, LI Yan, YIN Huifang, JIA Weixin. Establishment and Validation of the Reverse Genetics System for G57 Genotype H9N2 Subgenotype Avian Influenza Virus [J]. Acta Veterinaria et Zootechnica Sinica, 2025, 56(4): 1825-1833. |
| [2] | GAO Zhiqiang, LAI Ping'an, SONG Yueqian, CHONG Yan, GUO Youran, BAI Zilong, GUO Huimin, WANG Lin, PU Jing, SHI Xiju, REN Tong, ZHAO Xiangpeng. Studies and Application of Multi-target Nucleic Acid Mass Spectrometry Detection Method for Avian Influenza/Newcastle Disease Virus [J]. Acta Veterinaria et Zootechnica Sinica, 2025, 56(3): 1386-1395. |
| [3] | LU Na, GAO Yu, ZHAO Jiawei, SU Di, CHEN Jialei, LUO Zhongli. Construction and Characterization of Transcription Vectors for Feline Infectious Peritonitis Virus mRNA Vaccines [J]. Acta Veterinaria et Zootechnica Sinica, 2025, 56(2): 803-813. |
| [4] | Kangning ZHAO, Zhonglong YANG, Yi CHEN, Chuncheng ZHU, Yunfei GUO, Yuncong YIN, Tao QIN, Sujuan CHEN, Daxin PENG. Genetic Variation Analysis of Sixteen Novel H3N3 Subtype Avian Influenza Viruses [J]. Acta Veterinaria et Zootechnica Sinica, 2024, 55(9): 4029-4040. |
| [5] | MAO Qiuyan, ZHOU Shuning, LIU Shuo, PENG Cheng, YIN Xin, ZHANG Yaxin, ZHOU Wanting, LI Jinping, HOU Guangyu, JIANG Wenming, SONG Houhui, LIU Hualei. Establishment and Application of Fluorescent Quantitative RT-PCR for Detection of H3 Subtype Avian Influenza Virus [J]. Acta Veterinaria et Zootechnica Sinica, 2024, 55(3): 1137-1146. |
| [6] | YANG Zhiyi, WANG Xinkai, SHI Yuting, FU Siyuan, ZHANG Yuxin, CAO Chenfu, JIA Weixin. Establishment of Nucleic Acid Detection Methods for Avian Influenza H5 Subtype Based on CRISPR-Cas13a and RT-RAA [J]. Acta Veterinaria et Zootechnica Sinica, 2023, 54(9): 3803-3811. |
| [7] | CHEN Xin, QIN Tong. mRNA Vaccine and Its Research Prospect in Zoonotic Diseases [J]. Acta Veterinaria et Zootechnica Sinica, 2023, 54(7): 2732-2742. |
| [8] | ZHOU Yong, LI Zhixin, LU Hongwei, SUN Yan, LI Tian, DU Fanshu, PU Juan. Surveillance and Outbreak Analysis of H5 and H7N9 Subtypes of Highly Pathogenic Avian Influenza in China [J]. Acta Veterinaria et Zootechnica Sinica, 2022, 53(9): 3093-3106. |
| [9] | CHEN Huixian, CHEN Yajie, WANG Xianmei, WANG Lifang, LIU Qun, LIU Jing. Identification of the Cross-reacting Antigen MIC17A of Toxoplasma gondii and Neospora caninum and the Study of Its Cross-immune Protective Efficacy in Mice [J]. Acta Veterinaria et Zootechnica Sinica, 2022, 53(7): 2300-2306. |
| [10] | CUI Mingxian, WANG Xingbo, HUANG Yanming, BIAN Xiyi, FENG Mengke, YAN Yan, DONG Weiren, ZHOU Jiyong. Genetic Characterization and Evolution of Three Strains of H3N2 Avian Influenza Viruses [J]. Acta Veterinaria et Zootechnica Sinica, 2022, 53(11): 4116-4122. |
| [11] | LI Tian, YANG Yang, XIE Liqing, WANG Yuanlan, LI Pan, PENG Yuanyi, LI Nengzhang. Study on the Immunoprotection of Inactivated Vaccine of Bovine Mannheimia haemolytica and Bovine Pasteurella multocida Capsular Serotype A in Mouse Model [J]. Acta Veterinaria et Zootechnica Sinica, 2021, 52(9): 2579-2588. |
| [12] | LIU Junwen, WU Qiongjuan, XING Gang, ZHAN Songhe, WEI Jianzhong, SUN Pei, LIU Xuelan, LI Yu. Immunogenicity Analysis and Protective Effects of CbpB Protein of Erysipelothrix rhusiopathiae in Mice [J]. Acta Veterinaria et Zootechnica Sinica, 2021, 52(6): 1689-1699. |
| [13] | LI Jingyun, LIAN Pengjing, BAI Yu, XI Liuqing, ZHANG Zihui, NIU Xiaofei, YANG Junqi, QIAO Jian. The Impact of H9N2 Subtype Avian Influenza Viral Infection on the Gut Flora in Mice [J]. Acta Veterinaria et Zootechnica Sinica, 2021, 52(5): 1359-1368. |
| [14] | LI Li, TANG Guoyi, FENG Helong, XUE Yuhan, REN Zhu, WANG Guokang, JIA Miaomiao, SHANG Yu, LUO Qingping, SHAO Huabin, WEN Guoyuan. Evaluation of Immune Efficacy of H9 Subtype Avian Influenza Virus Inactivated Vaccine Based on Mosaic HA Sequence [J]. Acta Veterinaria et Zootechnica Sinica, 2021, 52(12): 3569-3577. |
| [15] | XU Lei, ZHONG Jialian, YU Xunxin, LIU Yifa, HUANG Yu, LIU Xiaolong, LAI Longyong, YAN Liping, XU Xiumei, SONG Suquan, ZHANG Yuankui. Improvement of the Immune Protection Rate on Newcastle Disease Virus Attenuated Vaccine LaSota Strain by Pig Spleen Transfer Factor [J]. Acta Veterinaria et Zootechnica Sinica, 2021, 52(12): 3578-3587. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||