





Acta Veterinaria et Zootechnica Sinica ›› 2025, Vol. 56 ›› Issue (3): 1006-1018.doi: 10.11843/j.issn.0366-6964.2025.03.004
• Review • Previous Articles Next Articles
WANG Xinyi1( ), YAO Junhu1, ZHANG Xia2, ZHANG Jun1,3,*(
), YAO Junhu1, ZHANG Xia2, ZHANG Jun1,3,*( )
)
Received:2024-05-30
Online:2025-03-23
Published:2025-04-02
Contact:
ZHANG Jun
E-mail:wxinyi@nwafu.edu.cn;jzhang0701@nwafu.edu.cn
CLC Number:
WANG Xinyi, YAO Junhu, ZHANG Xia, ZHANG Jun. Advances in Effect and Mechanism of Bile Acids Regulating Animal Intestinal Health[J]. Acta Veterinaria et Zootechnica Sinica, 2025, 56(3): 1006-1018.

Fig. 1
Regulatory effect of bile acids in animal intestinal physical barrier(Created by the BioRender.com) Villi length, crypt depth, and tight junctions between intestinal epithelial cells are important indicators of the integrity of the physical barrier of the gut. Bile acids increase villi length and decrease crypt depth to promote normal intestinal cell proliferation and protect the intestinal mucosa from injury or infection; At the same time, the key enzyme, Myosin light-chain kinase (MLCK), regulates the tight junctions between intestinal epithelial cells, thereby maintaining the integrity of the physical barrier"
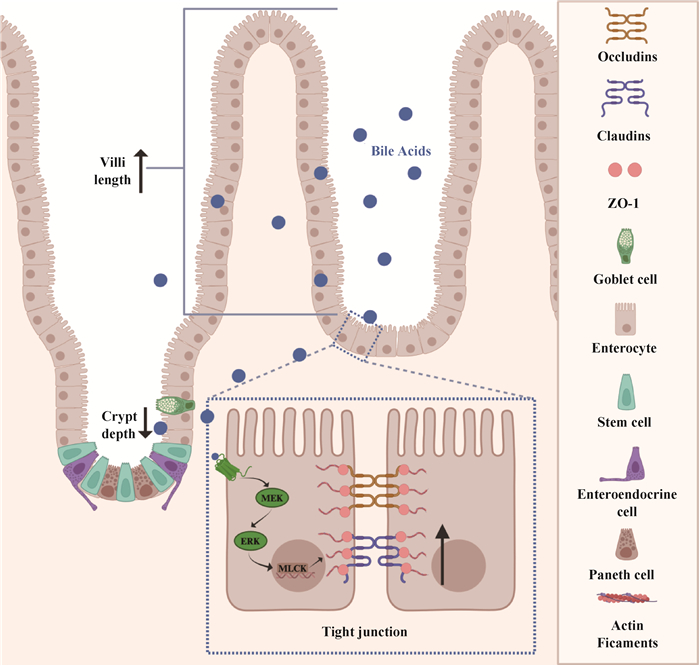
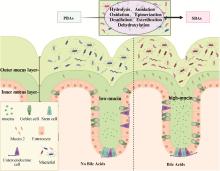
Fig. 2
Regulatory effect of bile acids on the intestinal mucus barrier and microbial barrier of animals (Created by the BioRender.com) The intestinal mucus barrier and the microbial barrier are closely interdependent and closely related. The intestinal mucus layer is divided into the inner mucus layer and the outer mucus layer, and the intestinal microbes mainly inhabit the outer mucus layer and are able to convert primary bile acids into secondary bile acids. On the contrary, after bile acids act on this barrier, the secretion of mucus protein increases significantly, and the structure of the microflora also changes significantly. PBAs: primary bile acids; SBAs: secondary bile acids"

Table 2
The modification effect of microbiota on BA"
| 转化作用/转化酶 Modification/Enzymes | 微生物生产者 Microbial producers | 转化及位点 Reactions and sites | 产物 Products | 参考文献 Reference |
| 水解作用/胆盐水解酶 Hydrolysis/Bile salt hydrolase | 拟杆菌门、厚壁菌门肠球菌、放线菌门 | -CONH转化COOH C24/RS | 非共轭胆汁酸 | [ |
| 脱羟基作用/胆酰辅酶 Dehydroxylation/Baioperonproteins | 厚壁菌门梭状芽孢杆菌属 | -OH转化-H C7 | 脱氧胆酸石胆酸 | [ |
| 氧化及差向异构化/羟基类固醇脱氢酶 Oxidation and Epimerization/ Hydroxysteroid dehydrogenase | 拟杆菌门、厚壁菌门变形菌门、放线菌门 | -OH转化=O和=O 转化-OH C3, C7, C12 | 熊脱氧胆汁酸胆汁酸含氧衍生物 | [ |
| 脱硫作用/硫酸酯酶 Desulfation/Sulfatase | 梭菌、消化球菌属、梭菌属变形菌门、假单胞菌属 | -SO3H2转化-OH C3 | 石胆酸、胆酸鹅脱氧胆酸 | [ |
| 酯化作用 Esterification | 拟杆菌门 | -OH转化-COOR C3和C24/RS | 3-乙酯 24-羰基酯 | [ |
| 酰胺化作用 Amidation | 拟杆菌、双歧杆菌肠球菌、肠杆菌 | -COOH转化-CONH-R C24/RS | 氨基酸偶联胆汁酸 | [ |

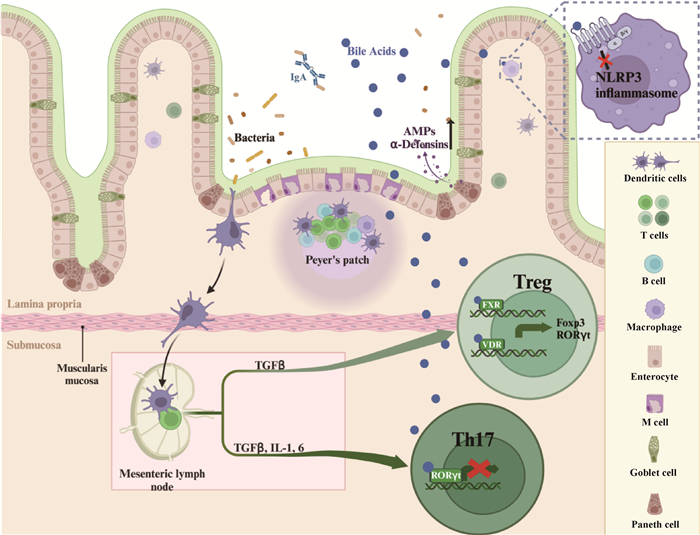
Fig. 3
Regulatory effect of bile acids in animal intestinal immune barrier(Created by the BioRender.com) The intestinal immune barrier is an important line of defense for animals against harmful pathogens. Bile acids can directly act on Paneth cells to promote the secretion of antimicrobial peptides such as α-defensin and promote macrophage polarization, and can also indirectly act on bile acid receptors such as FXR, VDR, RORγt, etc. to regulate the differentiation of helper T cells, and ultimately maintain the stability of the intestinal immune system. AMPs. Antimicrobial peptide; FXR. Farnesoid X-receptor; VDR. Vitamin D receptor; RORγt. Retinoic acid receptor-related orphan receptor γt"
| 1 |
CHIANG J , FERRELL J M . Bile acids as metabolic regulators and nutrient sensors[J]. Annu Rev Nutr, 2019, 39, 175- 200.
doi: 10.1146/annurev-nutr-082018-124344 |
| 2 | ZHANG J , ZHANG X , LIU H , et al. Altered bile acid and correlations with gut microbiome in transition dairy cows with different glucose and lipid metabolism status[J]. J Dairy Sci, 2024, |
| 3 |
PABOIS O , LORENZ C D , HARVEY R D , et al. Molecular insights into the behaviour of bile salts at interfaces: a key to their role in lipid digestion[J]. J Colloid Interface Sci, 2019, 556, 266- 277.
doi: 10.1016/j.jcis.2019.08.010 |
| 4 | URDANETA V , CASADESUS J . Interactions between bacteria and bile salts in the gastrointestinal and hepatobiliary tracts[J]. Front Med (Lausanne), 2017, 4, 163. |
| 5 |
FIORUCCI S , DISTRUTTI E , CARINO A , et al. Bile acids and their receptors in metabolic disorders[J]. Prog Lipid Res, 2021, 82, 101094.
doi: 10.1016/j.plipres.2021.101094 |
| 6 |
SHI L , JIN L , HUANG W . Bile acids, intestinal barrier dysfunction, and related diseases[J]. Cells, 2023, 12 (14): 1888.
doi: 10.3390/cells12141888 |
| 7 |
LI T , CHIANG J . Bile acids as metabolic regulators: an update[J]. Curr Opin Gastroenterol, 2023, 39 (3): 249- 255.
doi: 10.1097/MOG.0000000000000934 |
| 8 |
MACIERZANKA A , TORCELLO-GOMEZ A , JUNGNICKEL C , et al. Bile salts in digestion and transport of lipids[J]. Adv Colloid Interface Sci, 2019, 274, 102045.
doi: 10.1016/j.cis.2019.102045 |
| 9 |
YAN Y , LIU Y , ZENG C , et al. Effect of digestion on ursolic acid self-stabilized water-in-oil emulsion: Role of bile salts[J]. Foods, 2023, 12 (19): 3657.
doi: 10.3390/foods12193657 |
| 10 |
MULLISH B H , MCDONALD J , PECHLIVANIS A , et al. Microbial bile salt hydrolases mediate the efficacy of faecal microbiota transplant in the treatment of recurrent Clostridioides difficile infection[J]. Gut, 2019, 68 (10): 1791- 1800.
doi: 10.1136/gutjnl-2018-317842 |
| 11 |
ZHENG X , CHEN T , JIANG R , et al. Hyocholic acid species improve glucose homeostasis through a distinct TGR5 and FXR signaling mechanism[J]. Cell Metab, 2021, 33 (4): 791- 803.
doi: 10.1016/j.cmet.2020.11.017 |
| 12 |
BOUZAS C , PASTOR R , GARCIA S , et al. Comparative effects of glucagon-like peptide-1 receptors agonists, 4-dipeptidyl peptidase inhibitors, and metformin on metabolic syndrome[J]. Biomed Pharmacother, 2023, 161, 114561.
doi: 10.1016/j.biopha.2023.114561 |
| 13 |
VELAZQUEZ-VILLEGAS L A , PERINO A , LEMOS V , et al. TGR5 signalling promotes mitochondrial fission and beige remodelling of white adipose tissue[J]. Nat Commun, 2018, 9 (1): 245.
doi: 10.1038/s41467-017-02068-0 |
| 14 |
KRISHNAMURTHY H K , PEREIRA M , BOSCO J , et al. Gut commensals and their metabolites in health and disease[J]. Front Microbiol, 2023, 14, 1244293.
doi: 10.3389/fmicb.2023.1244293 |
| 15 |
VELAZQUEZ-VILLEGAS L A , PERINO A , LEMOS V , et al. TGR5 signalling promotes mitochondrial fission and beige remodelling of white adipose tissue[J]. Nat Commun, 2018, 9 (1): 245.
doi: 10.1038/s41467-017-02068-0 |
| 16 |
YE C , WU C , LI Y , et al. Traditional medicine Xianglian pill suppresses high-fat diet-related colorectal cancer via inactivating TLR4/MyD88 by remodeling gut microbiota composition and bile acid metabolism[J]. J Ethnopharmacol, 2024, 333, 118411.
doi: 10.1016/j.jep.2024.118411 |
| 17 |
WANG Q , SHEN W , SHAO W , et al. Berberine alleviates cholesterol and bile acid metabolism disorders induced by high cholesterol diet in mice[J]. Biochem Biophys Res Commun, 2024, 719, 150088.
doi: 10.1016/j.bbrc.2024.150088 |
| 18 | BAI X, DUAN Z, DENG J, et al. Ginsenoside Rh4 inhibits colorectal cancer via the modulation of gut microbiota-mediated bile acid metabolism[J/OL]. J Adv Res, (2024-07-03)[2024-05-30] https://www.sciencedirect.com/science/article/pii/S2090123224002650. |
| 19 | SUN B , XIE W , LI X , et al. Inulin enhanced rifaximin-inhibited colon cancer pulmonary metastasis by flora-regulated bile acid pathway[J]. Int J Biol Macromol, 2024, 275 (Pt 1): 133582. |
| 20 | ZHU Y , SUN G , CIDAN Y , et al. Comprehensive multi-omic evaluation of the microbiota and metabolites in the colons of diverse swine breeds[J]. Animals (Basel), 2024, 14 (8): 1221. |
| 21 | ROWE J C , WINSTON J A . Collaborative metabolism: Gut microbes play a key role in canine and feline bile acid metabolism[J]. Vet Sci, 2024, 11 (2): 94. |
| 22 |
YOUSEFI J , TAHERPOUR K , GHASEMI H A , et al. RETRACTED ARTICLE: Effects of emulsifier, betaine and L-carnitine on growth performance, immune response, gut morphology and nutrient digestibility in broiler chickens exposed to cyclic heat stress[J]. Br Poult Sci, 2023, 64 (4): iii- xvi.
doi: 10.1080/00071668.2022.2124100 |
| 23 |
ZAHID M U , KHALIQUE A , QAISRANI S N , et al. The effect of Acacia nilotica bark extract on growth performance, carcass characteristics, immune response, and intestinal morphology in broilers as an alternative to antibiotic growth promoter[J]. Anim Biosci, 2023, 36 (7): 1059- 1066.
doi: 10.5713/ab.22.0284 |
| 24 | GU Y F , CHEN Y P , JIN R , et al. Dietary chitooligosaccharide supplementation alleviates intestinal barrier damage, and oxidative and immunological stress in lipopolysaccharide-challenged laying hens[J]. Poult Sci, 2022, 101 (4): 101701. |
| 25 |
MARTEL J , CHANG S H , KO Y F , et al. Gut barrier disruption and chronic disease[J]. Trends Endocrinol Metab, 2022, 33 (4): 247- 265.
doi: 10.1016/j.tem.2022.01.002 |
| 26 |
JAIN A K , STOLL B , BURRIN D G , et al. Enteral bile acid treatment improves parenteral nutrition-related liver disease and intestinal mucosal atrophy in neonatal pigs[J]. Am J Physiol Gastrointest Liver Physiol, 2012, 302 (2): G218- G224.
doi: 10.1152/ajpgi.00280.2011 |
| 27 |
PERRONE E E , CHEN C , LONGSHORE S W , et al. Dietary bile acid supplementation improves intestinal integrity and survival in a murine model[J]. J Pediatr Surg, 2010, 45 (6): 1256- 1265.
doi: 10.1016/j.jpedsurg.2010.02.094 |
| 28 |
YANG J , VAN DIJK T H , KOEHORST M , et al. Intestinal farnesoid X receptor modulates duodenal surface area but does not control glucose absorption in mice[J]. Int J Mol Sci, 2023, 24 (4): 4132.
doi: 10.3390/ijms24044132 |
| 29 |
SONG J , LI Q , LI P , et al. The effects of inulin on the mucosal morphology and immune status of specific pathogen-free chickens[J]. Poult Sci, 2018, 97 (11): 3938- 3946.
doi: 10.3382/ps/pey260 |
| 30 |
RAIMONDI F , SANTORO P , BARONE M V , et al. Bile acids modulate tight junction structure and barrier function of Caco-2 monolayers via EGFR activation[J]. Am J Physiol Gastrointest Liver Physiol, 2008, 294 (4): G906- G913.
doi: 10.1152/ajpgi.00043.2007 |
| 31 |
YAO B , HE J , YIN X , et al. The protective effect of lithocholic acid on the intestinal epithelial barrier is mediated by the vitamin D receptor via a SIRT1/Nrf2 and NF-kappaB dependent mechanism in Caco-2 cells[J]. Toxicol Lett, 2019, 316, 109- 118.
doi: 10.1016/j.toxlet.2019.08.024 |
| 32 |
BUCKLEY A , TURNER J R . Cell biology of tight junction barrier regulation and mucosal disease[J]. Cold Spring Harb Perspect Biol, 2018, 10 (1): a029314.
doi: 10.1101/cshperspect.a029314 |
| 33 |
SONG M , YE J , ZHANG F , et al. Chenodeoxycholic acid (CDCA) protects against the lipopolysaccharide-induced impairment of the intestinal epithelial barrier function via the FXR-MLCK pathway[J]. J Agric Food Chem, 2019, 67 (32): 8868- 8874.
doi: 10.1021/acs.jafc.9b03173 |
| 34 |
SONG M , ZHANG F , FU Y , et al. Tauroursodeoxycholic acid (TUDCA) improves intestinal barrier function associated with TGR5-MLCK pathway and the alteration of serum metabolites and gut bacteria in weaned piglets[J]. J Anim Sci Biotechnol, 2022, 13 (1): 73.
doi: 10.1186/s40104-022-00713-3 |
| 35 |
RUAN D , WU S , FOUAD A M , et al. Curcumin alleviates LPS-induced intestinal homeostatic imbalance through reshaping gut microbiota structure and regulating group 3 innate lymphoid cells in chickens[J]. Food Funct, 2022, 13 (22): 11811- 11824.
doi: 10.1039/D2FO02598A |
| 36 |
DIEGO-CABERO N , MEREU A , MENOYO D , et al. Bile acid mediated effects on gut integrity and performance of early-weaned piglets[J]. BMC Vet Res, 2015, 11, 111.
doi: 10.1186/s12917-015-0425-6 |
| 37 |
SONG M , ZHANG F , CHEN L , et al. Dietary chenodeoxycholic acid improves growth performance and intestinal health by altering serum metabolic profiles and gut bacteria in weaned piglets[J]. Anim Nutr, 2021, 7 (2): 365- 375.
doi: 10.1016/j.aninu.2020.07.011 |
| 38 | VAN DER LUGT B , VOS M , GROOTTE B M , et al. The effects of sulfated secondary bile acids on intestinal barrier function and immune response in an inflammatory in vitro human intestinal model[J]. Heliyon, 2022, 8 (2): e8883. |
| 39 | LEE H Y , CRAWLEY S , HOKARI R , et al. Bile acid regulates MUC2 transcription in colon cancer cells via positive EGFR/PKC/Ras/ERK/CREB, PI3K/Akt/IkappaB/NF-kappaB and p38/MSK1/CREB pathways and negative JNK/c-Jun/AP-1 pathway[J]. Int J Oncol, 2010, 36 (4): 941- 953. |
| 40 |
SANTOS G M , ISMAEL S , MORAIS J , et al. Intestinal alkaline phosphatase: A review of this enzyme role in the intestinal barrier function[J]. Microorganisms, 2022, 10 (4): 746.
doi: 10.3390/microorganisms10040746 |
| 41 |
GUPTA U , DEY P . Rise of the guardians: Gut microbial maneuvers in bacterial infections[J]. Life Sci, 2023, 330, 121993.
doi: 10.1016/j.lfs.2023.121993 |
| 42 |
IANCU M A , PROFIR M , ROSU O A , et al. Revisiting the intestinal microbiome and its role in diarrhea and constipation[J]. Microorganisms, 2023, 11 (9): 2177.
doi: 10.3390/microorganisms11092177 |
| 43 |
CAI J , SUN L , GONZALEZ F J . Gut microbiota-derived bile acids in intestinal immunity, inflammation, and tumorigenesis[J]. Cell Host Microbe, 2022, 30 (3): 289- 300.
doi: 10.1016/j.chom.2022.02.004 |
| 44 |
CHIANG J Y . Bile acid metabolism and signaling[J]. Compr Physiol, 2013, 3 (3): 1191- 1212.
doi: 10.1002/j.2040-4603.2013.tb00517.x |
| 45 |
MOHANTY I , MANNOCHIO-RUSSO H , SCHWEER J V , et al. The underappreciated diversity of bile acid modifications[J]. Cell, 2024, 187 (7): 1801- 1818.
doi: 10.1016/j.cell.2024.02.019 |
| 46 |
WANG Y Z , MEI P C , BAI P R , et al. A strategy for screening and identification of new amino acid-conjugated bile acids with high coverage by liquid chromatography-mass spectrometry[J]. Anal Chim Acta, 2023, 1239, 340691.
doi: 10.1016/j.aca.2022.340691 |
| 47 | KISTHARDT S C , THANISSERY R , PIKE C M , et al. The microbial-derived bile acid lithocholate and its epimers inhibit Clostridioides difficile growth and pathogenicity while sparing members of the gut microbiota[J]. J Bacteriol, 2023, 205 (9): e18023. |
| 48 |
DAHIYA M , JOVEL J , MONAGHAN T , et al. In silico analysis of changes in predicted metabolic capabilities of intestinal microbiota after fecal microbial transplantation for treatment of recurrent Clostridioides difficile Infection[J]. Microorganisms, 2023, 11 (4): 1078.
doi: 10.3390/microorganisms11041078 |
| 49 |
DODEN H L , RIDLON J M . Microbial hydroxysteroid dehydrogenases: From alpha to omega[J]. Microorganisms, 2021, 9 (3): 469.
doi: 10.3390/microorganisms9030469 |
| 50 |
ZHU Q F , WANG Y Z , AN N , et al. Alternating dual-collision energy scanning mass spectrometry approach: Discovery of novel microbial bile-acid conjugates[J]. Anal Chem, 2022, 94 (5): 2655- 2664.
doi: 10.1021/acs.analchem.1c05272 |
| 51 |
FU T , HUAN T , RAHMAN G , et al. Paired microbiome and metabolome analyses associate bile acid changes with colorectal cancer progression[J]. Cell Rep, 2023, 42 (8): 112997.
doi: 10.1016/j.celrep.2023.112997 |
| 52 |
PAIK D , YAO L , ZHANG Y , et al. Human gut bacteria produce Tau(Eta)17-modulating bile acid metabolites[J]. Nature, 2022, 603 (7903): 907- 912.
doi: 10.1038/s41586-022-04480-z |
| 53 |
ZHANG X , YUN Y , LAI Z , et al. Supplemental Clostridium butyricum modulates lipid metabolism by reshaping the gut microbiota composition and bile acid profile in IUGR suckling piglets[J]. J Anim Sci Biotechnol, 2023, 14 (1): 36.
doi: 10.1186/s40104-023-00828-1 |
| 54 |
ELKINS C A , MOSER S A , SAVAGE D C . Genes encoding bile salt hydrolases and conjugated bile salt transporters in Lactobacillus johnsonii 100-100 and other Lactobacillus species[J]. Microbiology (Reading), 2001, 147 (12): 3403- 3412.
doi: 10.1099/00221287-147-12-3403 |
| 55 |
TANAKA H , HASHIBA H , KOK J , et al. Bile salt hydrolase of Bifidobacterium longum-biochemical and genetic characterization[J]. Appl Environ Microbiol, 2000, 66 (6): 2502- 2512.
doi: 10.1128/AEM.66.6.2502-2512.2000 |
| 56 |
WIJAYA A , HERMANN A , ABRIOUEL H , et al. Cloning of the bile salt hydrolase (bsh) gene from Enterococcus faecium FAIR-E 345 and chromosomal location of bsh genes in food enterococci[J]. J Food Prot, 2004, 67 (12): 2772- 2778.
doi: 10.4315/0362-028X-67.12.2772 |
| 57 |
KITAHARA M , TAKAMINE F , IMAMURA T , et al. Assignment of Eubacterium sp, VPI 12708 and related strains with high bile acid 7alpha-dehydroxylating activity to Clostridium scindens and proposal of Clostridium hylemonae sp. nov., isolated from human faeces[J]. Int J Syst Evol Microbiol, 2000, 50 (3): 971- 978.
doi: 10.1099/00207713-50-3-971 |
| 58 |
KITAHARA M , TAKAMINE F , IMAMURA T , et al. Clostridium hiranonis sp. nov., a human intestinal bacterium with bile acid 7alpha-dehydroxylating activity[J]. Int J Syst Evol Microbiol, 2001, 51 (1): 39- 44.
doi: 10.1099/00207713-51-1-39 |
| 59 |
BURNS D A , HEAP J T , MINTON N P . SleC is essential for germination of Clostridium difficile spores in nutrient-rich medium supplemented with the bile salt taurocholate[J]. J Bacteriol, 2010, 192 (3): 657- 664.
doi: 10.1128/JB.01209-09 |
| 60 | HARRIS S C , DEVENDRAN S , MENDEZ-GARCIA C , et al. Bile acid oxidation by Eggerthella lenta strains C592 and DSM 2243(T)[J]. Gut Microbes, 2018, 9 (6): 523- 539. |
| 61 |
EDENHARDER R , PFUTZNER A , HAMMANN R . Characterization of NAD-dependent 3 alpha- and 3 beta-hydroxysteroid dehydrogenase and of NADP-dependent 7 beta-hydroxysteroid dehydrogenase from Peptostreptococcus productus[J]. Biochim Biophys Acta, 1989, 1004 (2): 230- 238.
doi: 10.1016/0005-2760(89)90272-5 |
| 62 | DODEN H , SALLAM L A , DEVENDRAN S , et al. Metabolism of Oxo-bile acids and characterization of recombinant 12alpha-hydroxysteroid dehydrogenases from bile acid 7alpha-dehydroxylating human gut bacteria[J]. Appl Environ Microbiol, 2018, 84 (10): e00235- 18. |
| 63 |
WANG P , CHEN Q , YUAN P , et al. Gut microbiota involved in desulfation of sulfated progesterone metabolites: A potential regulation pathway of maternal bile acid homeostasis during pregnancy[J]. Front Microbiol, 2022, 13, 1023623.
doi: 10.3389/fmicb.2022.1023623 |
| 64 |
JIANG M , XU M , REN S , et al. Transgenic overexpression of steroid sulfatase alleviates cholestasis[J]. Liver Res, 2017, 1 (1): 63- 69.
doi: 10.1016/j.livres.2017.03.001 |
| 65 |
ROBBEN J , JANSSEN G , MERCKX R , et al. Formation of delta 2- and delta 3-cholenoic acids from bile acid 3-sulfates by a human intestinal Fusobacterium strain[J]. Appl Environ Microbiol, 1989, 55 (11): 2954- 2959.
doi: 10.1128/aem.55.11.2954-2959.1989 |
| 66 | BURCHAT N , VIDOLA J , PFREUNDSCHUH S , et al. Intestinal stearoyl-CoA desaturase-1 regulates energy balance via alterations in bile acid homeostasis[J]. bioRxiv, 2024, 12, 575400. |
| 67 |
SONG X , SUN X , OH S F , et al. Microbial bile acid metabolites modulate gut RORgamma(+) regulatory T cell homeostasis[J]. Nature, 2020, 577 (7790): 410- 415.
doi: 10.1038/s41586-019-1865-0 |
| 68 |
QUINN R A , MELNIK A V , VRBANAC A , et al. Global chemical effects of the microbiome include new bile-acid conjugations[J]. Nature, 2020, 579 (7797): 123- 129.
doi: 10.1038/s41586-020-2047-9 |
| 69 |
DEVLIN A S , FISCHBACH M A . A biosynthetic pathway for a prominent class of microbiota-derived bile acids[J]. Nat Chem Biol, 2015, 11 (9): 685- 690.
doi: 10.1038/nchembio.1864 |
| 70 |
WATANABE M , FUKIYA S , YOKOTA A . Comprehensive evaluation of the bactericidal activities of free bile acids in the large intestine of humans and rodents[J]. J Lipid Res, 2017, 58 (6): 1143- 1152.
doi: 10.1194/jlr.M075143 |
| 71 |
INAGAKI T , MOSCHETTA A , LEE Y K , et al. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor[J]. Proc Natl Acad Sci U S A, 2006, 103 (10): 3920- 3925.
doi: 10.1073/pnas.0509592103 |
| 72 |
GADALETA R M , GARCIA-IRIGOYEN O , CARIELLO M , et al. Fibroblast Growth Factor 19 modulates intestinal microbiota and inflammation in presence of Farnesoid X Receptor[J]. EBioMedicine, 2020, 54, 102719.
doi: 10.1016/j.ebiom.2020.102719 |
| 73 |
PI Y , WU Y , ZHANG X , et al. Gut microbiota-derived ursodeoxycholic acid alleviates low birth weight-induced colonic inflammation by enhancing M2 macrophage polarization[J]. Microbiome, 2023, 11 (1): 19.
doi: 10.1186/s40168-022-01458-x |
| 74 |
WANG S , DONG W , LIU L , et al. Interplay between bile acids and the gut microbiota promotes intestinal carcinogenesis[J]. Mol Carcinog, 2019, 58 (7): 1155- 1167.
doi: 10.1002/mc.22999 |
| 75 |
XU M , CEN M , SHEN Y , et al. Deoxycholic Acid-Induced Gut Dysbiosis Disrupts Bile Acid Enterohepatic Circulation and Promotes Intestinal Inflammation[J]. Dig Dis Sci, 2021, 66 (2): 568- 576.
doi: 10.1007/s10620-020-06208-3 |
| 76 |
YIN Q , YU J , LI J , et al. Enhancing milk quality and modulating rectal microbiota of dairy goats in starch-rich diet: the role of bile acid supplementation[J]. J Anim Sci Biotechnol, 2024, 15 (1): 7.
doi: 10.1186/s40104-023-00957-7 |
| 77 |
YANG B , HUANG S , ZHAO G , et al. Dietary supplementation of porcine bile acids improves laying performance, serum lipid metabolism and cecal microbiota in late-phase laying hens[J]. Anim Nutr, 2022, 11, 283- 292.
doi: 10.1016/j.aninu.2022.08.003 |
| 78 |
GONG T , LIU L , JIANG W , et al. DAMP-sensing receptors in sterile inflammation and inflammatory diseases[J]. Nat Rev Immunol, 2020, 20 (2): 95- 112.
doi: 10.1038/s41577-019-0215-7 |
| 79 |
CHE Y , XU W , DING C , et al. Bile acids target mitofusin 2 to differentially regulate innate immunity in physiological versus cholestatic conditions[J]. Cell Rep, 2023, 42 (1): 112011.
doi: 10.1016/j.celrep.2023.112011 |
| 80 |
WANG Y , YU Y , LI L , et al. Bile acid-dependent transcription factors and chromatin accessibility determine regional heterogeneity of intestinal antimicrobial peptides[J]. Nat Commun, 2023, 14 (1): 5093.
doi: 10.1038/s41467-023-40565-7 |
| 81 | TREMBLAY S , ROMAIN G , ROUX M , et al. Bile acid administration elicits an intestinal antimicrobial program and reduces the bacterial burden in two mouse models of enteric infection[J]. Infect Immun, 2017, 85 (6): e00942- 16. |
| 82 |
LIU T C , KERN J T , JAIN U , et al. Western diet induces Paneth cell defects through microbiome alterations and farnesoid X receptor and type I interferon activation[J]. Cell Host Microbe, 2021, 29 (6): 988- 1001.
doi: 10.1016/j.chom.2021.04.004 |
| 83 | 廖楚瑶, 李思奇, 张尊建, 等. 肠道疾病中胆汁酸及其受体对NLRP3炎症小体调控作用的研究进展[J]. 药学进展, 2022, 46 (3): 218- 225. |
| LIAO C Y , LI S Q , ZHANG Z J , et al. Research progress in the regulatory of bile acids and bile acids-activated receptors on NLRP3 inflammasome in intestinal diseases[J]. Advances in Pharmacy, 2022, 46 (3): 218- 225. | |
| 84 | 聂源. 基于肠道菌-肝胆汁酸轴探究熊果酸抑制肝纤维化的作用机制[D]. 南昌: 南昌大学, 2022. |
| NIE Y. Exploring the mechanism of ursolic acid in inhibiting liver fibrosis based on the intestinal microbiota-hepatobiliac axis[D]. Nanchang: Nanchang University, 2022. (in Chinese) | |
| 85 |
HANG S , PAIK D , YAO L , et al. Bile acid metabolites control T(H)17 and T(reg) cell differentiation[J]. Nature, 2019, 576 (7785): 143- 148.
doi: 10.1038/s41586-019-1785-z |
| 86 |
DONG X , QI M , CAI C , et al. Farnesoid X receptor mediates macrophage-intrinsic responses to suppress colitis-induced colon cancer progression[J]. JCI Insight, 2024, 9 (2): e170428.
doi: 10.1172/jci.insight.170428 |
| 87 |
XING J H , NIU T M , ZOU B S , et al. Gut microbiota-derived LCA mediates the protective effect of PEDV infection in piglets[J]. Microbiome, 2024, 12 (1): 20.
doi: 10.1186/s40168-023-01734-4 |
| 88 |
PENG X R , FENG L , JIANG W D , et al. Supplementation exogenous bile acid improved growth and intestinal immune function associated with NF-kappaB and TOR signalling pathways in on-growing grass carp (Ctenopharyngodon idella): Enhancement the effect of protein-sparing by dietary lipid[J]. Fish Shellfish Immunol, 2019, 92, 552- 569.
doi: 10.1016/j.fsi.2019.06.047 |
| 89 |
KUBOTA H , ISHIZAWA M , KODAMA M , et al. Vitamin D receptor mediates attenuating fffect of lithocholic acid on dextran sulfate sodium induced colitis in mice[J]. Int J Mol Sci, 2023, 24 (4): 3517.
doi: 10.3390/ijms24043517 |
| 90 |
LEE G R . The balance of Th17 versus treg cells in autoimmunity[J]. Int J Mol Sci, 2018, 19 (3): 730.
doi: 10.3390/ijms19030730 |
| [1] | BAI Guosong, TENG Chunran, WANG Junhong, ZHONG Ruqing, MA Teng, CHEN Liang, ZHANG Hongfu. Effects of Enzymatic Corn Gluten Meal on Growth Performance and Intestinal Microorganisms of Weaned Piglets [J]. Acta Veterinaria et Zootechnica Sinica, 2025, 56(2): 953-968. |
| [2] | Xiuju YU, Yanjiao HU, Jiayue LIU, Haidong WANG, Zhiwei ZHU, Kuohai FAN, Rongrong WANG, Chenghao DUAN, Jiawei SHI, Lihua YANG. Isolation and Identification of a Chicken Source Lactobacillus salivary Strain and Its Effect on Intestinal Health of Laying Hens in Early Brood Period [J]. Acta Veterinaria et Zootechnica Sinica, 2024, 55(9): 4161-4171. |
| [3] | Yu CHEN, Ziqing XIU, Musa MGENI, Yi SHI, Junqiu ZHANG, Xiaoyu JIANG, Jingzhi LÜ, Yawang SUN. Effects of Dandelion and Akebia Extract on Growth Performance, Intestinal Health and Relative Expression of Drug Transporter Genes in Weaned Rabbits [J]. Acta Veterinaria et Zootechnica Sinica, 2024, 55(8): 3725-3739. |
| [4] | Yalin LI, Shibo ZHEN, Lin CAO, Fengxue SUN, Lihua WANG. Effects of Lactobacillus plantarum and Lactobacillus plantarum Postbiotics on Growth Performance, Immune Status and Intestinal Health of Growing Female Minks [J]. Acta Veterinaria et Zootechnica Sinica, 2024, 55(6): 2530-2539. |
| [5] | HAN Fuzhen, CAI Limeng, LI Zhuoran, WANG Xueying, XIE Weichun, KUANG Hongdi, LI Jiaxuan, CUI Wen, JIANG Yanping, LI Yijing, SHAN Zhifu, TANG Lijie. Research Progress on the Mechanism of Intestinal Flora-Mediated Regulation of Intestinal Mucosal Immunity by Secondary Bile Acids and Their Receptors [J]. Acta Veterinaria et Zootechnica Sinica, 2024, 55(5): 1904-1913. |
| [6] | NIU Xiaoyu, XING Yuanyuan, LI Dabiao. Advances in Regulation and Mechanism of Plant Bioactive Compounds on Intestinal Barrier Function in Animals [J]. Acta Veterinaria et Zootechnica Sinica, 2024, 55(4): 1467-1477. |
| [7] | LI Tie, QI Mengdi, ZHANG Keying, WANG Jianping, BAI Shiping, ZENG Qiufeng, PENG Huanwei, XUAN Yue, LÜ Li, DING Xuemei. Effects of Dietary Probiotics Supplementation during Brood-rearing Period on Growth Performance, Serum Biochemistry, Intestinal Health and Subsequent Performance of Laying Hens [J]. Acta Veterinaria et Zootechnica Sinica, 2024, 55(3): 1062-1076. |
| [8] | Daoliang ZHANG, Hongyan DING, Liuxing WANG, Wenjun TAI, Hao KONG, Chang ZHAO, Shibin FENG, Xichun WANG, Yanfeng XUE, Jinjie WU, Yu LI. Effect of Rumen Acidosis on Gastrointestinal Function, Morphology, and Microflora in Goats [J]. Acta Veterinaria et Zootechnica Sinica, 2024, 55(10): 4760-4772. |
| [9] | MU Xiangyu, XU Yunruo, HU Jingyi, ZHOU Xinyan, ZHU Yongwen. Advances in Research on the Nutritional Requirements of Branched-Chain Amino Acids in Poultry [J]. Acta Veterinaria et Zootechnica Sinica, 2024, 55(1): 31-38. |
| [10] | LUO Ju, MAO Jiani, XIA Yinzhao, YANG Zhenguo. Regulation of circRNAs on Mammalian Intestinal Barrier Function [J]. Acta Veterinaria et Zootechnica Sinica, 2023, 54(11): 4439-4448. |
| [11] | WU Diange, XIA Miao, YAN An, JIANG Haotian, FAN Jiaqi, ZHOU Siyuan, WEI Xu, LIU Shudong, CHEN Baojiang. Effects of Carvacrol on Growth Performance, Nutrient Apparent Digestibility, Intestinal Morphology, Short-chain Fatty Acids Content and Intestinal Flora in Rabbits [J]. Acta Veterinaria et Zootechnica Sinica, 2023, 54(10): 4233-4246. |
| [12] | YUAN Tong, HUANG Liang, YANG Lin, WANG Wence, ZHU Yongwen. Regulation of Mitochondrial Function by Gut Microbiota and Their Metabolites in Animal [J]. Acta Veterinaria et Zootechnica Sinica, 2023, 54(1): 48-57. |
| [13] | DU Xue'er, WANG Jing, YAO Junhu, CAO Yangchun. Bile Acid Enterohepatic Circulation Transporter and Its Regulatory Mechanism by FXR [J]. Acta Veterinaria et Zootechnica Sinica, 2021, 52(10): 2721-2739. |
| [14] | AN Qing-cong,XU Na-na,ZHANG Chun-yong,PAN Hong-bin,LI Mei-quan,CHEN Ke-lin,GUO Rong-fu. The Effect of Different Levels of Lactoferrin on the Growth Performance,Small Intestinal Morphology and Body Resistance to Disease of Diansa Weaning Piglets [J]. ACTA VETERINARIA ET ZOOTECHNICA SINICA, 2015, 46(12): 2206-2217. |
| [15] | LI Qing-zhu,LI Run-hang,ZHENG Yan-qiu,LOU Yu-jie. Effect of Different Sources of Fiber on Intestinal Morphological Development of Jilin White Geese [J]. ACTA VETERINARIA ET ZOOTECHNICA SINICA, 2014, 45(4): 596-602. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||