





Acta Veterinaria et Zootechnica Sinica ›› 2024, Vol. 55 ›› Issue (9): 3897-3913.doi: 10.11843/j.issn.0366-6964.2024.09.015
• Animal Genetics and Breeding • Previous Articles Next Articles
Zijin YUAN( ), Wanxin WANG, Ya XING, Jiahui LI, Ying XUE, Jing GE, Minmeng ZHAO, Long LIU, Daoqing GONG, Tuoyu GENG*(
), Wanxin WANG, Ya XING, Jiahui LI, Ying XUE, Jing GE, Minmeng ZHAO, Long LIU, Daoqing GONG, Tuoyu GENG*( )
)
Received:2024-02-18
Online:2024-09-23
Published:2024-09-27
Contact:
Tuoyu GENG
E-mail:2316066266@qq.com;tygeng@yzu.edu.cn
CLC Number:
Zijin YUAN, Wanxin WANG, Ya XING, Jiahui LI, Ying XUE, Jing GE, Minmeng ZHAO, Long LIU, Daoqing GONG, Tuoyu GENG. HDLBP Is Involved in Goose Fatty Liver Formation by Regulating the Level of Oxidative Stress and the Expression of Inflammatory Factors[J]. Acta Veterinaria et Zootechnica Sinica, 2024, 55(9): 3897-3913.
Table 1
The sequence of primers for qRT-PCR"
| 基因 Gene | 引物序列(5′→3′) Primers sequence | 产物大小/bp Product size |
| HDLBP | F: CCGTGGAGGTGAAGAAGTCC; R: CCTCGCAGTATCACCGTCTC | 139 |
| UBC | F: AGGGTGGATTCTTTCTGG; R: ACTGAGTTTGGAGGGAGC | 243 |
| GAPDH | F: CTGATGCTCCCATGTTCGT; R: CCACGATGCCAAAGTTGTCA | 138 |
| CHP1 | F: TCCAGAAGGAGAGGACCAAGT; R: GGAGCTTGTTACTTCGGCTG | 134 |
| PCAN1 | F: TTGCCTGTGTGGCAGATAGT; R: TGTGCAACTGGACTCTAGCAT | 177 |
| LOC106049357 | F: TGGCAGATGCAGACACAACC; R: CACCCTGGCCTCTCGTAAAT | 140 |
| HRG | F: ACAAGGGATTCTGCAAGGCAC; R: TCTATGTCCATGGTGATGCTGCC | 107 |
| ELOVL6 | F: GGTGGTCGGCACCTAATGAA; R: TCTGGTCACACACTGACTGC | 169 |
| GMPR | F: CTGCCAATCACCCAGAATGC; R: TCAGAGTAGCCGTTTGCCAC | 142 |
| ALDH1A1 | F: GGCGACAAGGCAGATGTAGA; R: CAAGAGCCTTCCTCGCTCTG | 105 |
| MAT1A | F: TGCGCGTTCATACCATTGTG; R: GACCAGTAACACCAGCGTCA | 198 |
| PSAT1 | F: AGAATGTTGGCTGTGCTGGA; R: AAAGAGCCATTCACCGCTTG | 118 |
| LOC106034448 | F: ATCTGCTTGCTCCCTGGATT; R: TGTCCCTTTTCCATCTGCCA | 187 |
| SLC2A9 | F: GCAGGGAAGGGCAATTGGT; R: GACCAGAGGAGAGTCAGGGT | 194 |
| IL1R1 | F: GAACGCCCAGCTCAGAACAT; R: AAAGGATGGCACGAGTTCCG | 140 |
| LTC4S | F: TTCGAGCACAGGTGAACTCC; R: GAGGACGATGCCCTGTATCC | 157 |
| TNFSF10 | F: TGTCCACAGGATAGCAGCAC; R: GGTATCACCAGCTCGCCATT | 180 |
| NCF1 | F: GACACCTTCATCCGGCACA; R: TTTCGGTGAGGTCGTTCCAC | 106 |
| SFTPA1 | F: GGCACACCTCTGAATTACACC; R: TGTGAGGCGATACAGGTTGC | 126 |
| KDR | F: ACCTGACGATGAACCCACAC; R: CCACATTCAGCTCCGTCCTT | 90 |
| PPAP2B | F: TGCTATCATCACGGGAGAGC; R: TGTGAAGGACTGGCTGATGG | 142 |
| SIGLEC1 | F: CTTCAGCACCCAAAAGACCG; R: CAGGACATCCGAAACGCTCA | 229 |
| CCL20 | F: TGAACAGCTCTCCAGTGAAGTC; R: TCCTTAGGGTTTACGCAGGC | 87 |

Fig. 2
Co-localization of HDLBP protein and mitochondria in goose primary hepatocytes A. The immunofluorescence image, DAPI indicates the nucleus, Mito-tracker Red indicates mitochondria, and yellow in the merged image indicates co-localization of HDLBP protein with mitochondrial; B. The immunoblotting image (n=3); C. The quantification of immunoblotting, the letter "M" represents protein marker, β-Actin serves as an internal reference gene in the cytoplasm, while VDAC serves as an internal reference gene in mitochondria. *. P < 0.05, the same as below"


Fig. 3
The relative amount of HDLBP protein in whole cell and mitochondrial lysates of the livers of Landes geese in the overfeeding and control groups A. The immunoblotting image of whole cell lysate (n=6); B. The quantification of immunoblotting of whole cell lysate; C. The immunoblotting image of mitochondrial lysate (n=4); D. The quantification of immunoblotting of mitochondrial lysate. The letter "C" represents the control group, the letter "T" represents the overfeeding group, GAPDH and VDAC are used as the internal reference gene. **. P < 0.01, the same as below"


Fig. 4
Detection of HDLBP overexpression in goose primary hepatocytes A. Detection of HDLBP mRNA level by quantitative PCR (n=6); B. Detection of HDLBP protein level by immunoblotting (n=4); C. The quantification of immunoblotting. The letter "C" represents the control group, while "OE" represents the overexpression group. ***. P < 0.001, the same as below"


Fig. 5
Effects of HDLBP overexpression on mitochondrial function in primary goose hepatocytes A. Detection of the level of reactive oxygen species by flow cytometry; B. Quantitative analysis using FlowJo software; C. Detection of mitochondrial membrane potential by flow cytometry; D. Quantitative analysis using FlowJo software, n=3; E. The level of MDA; F. The activity of T-SOD; G. The activity of GSH-PX, n=6"
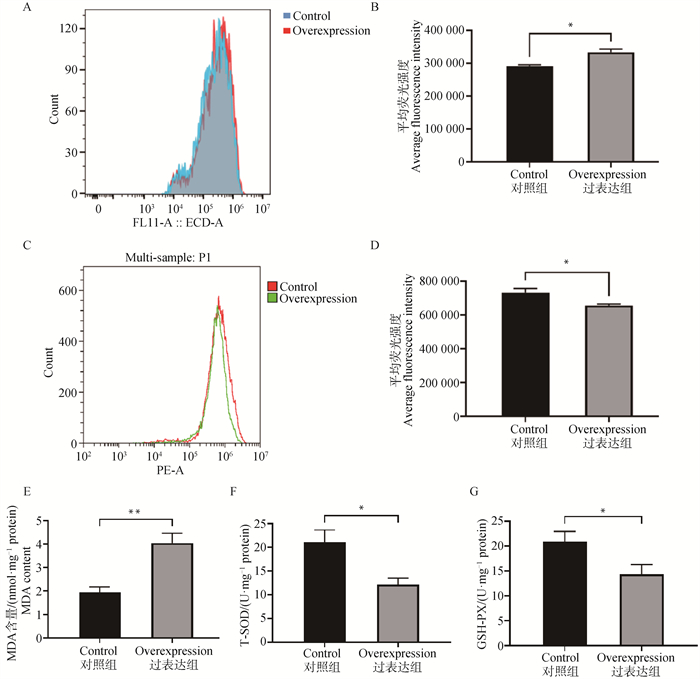
Fig. 6
The volcano map (A) and the clustering heat map (B) of the differentially expressed genes (DEGs) A. The volcano map of DEGs, up-regulated genes are indicated by red dots, down-regulated genes are indicated by green dots, and the gray dashed line indicates the threshold line of screening criteria for DEGs; B. The clustering heat map of DEGs, X-axis indicates sample names, Y-axis indicates the normalized FPKM values of DEGs, the darker the red color indicates the higher expression level, and the darker the blue color indicates the lower expression level"
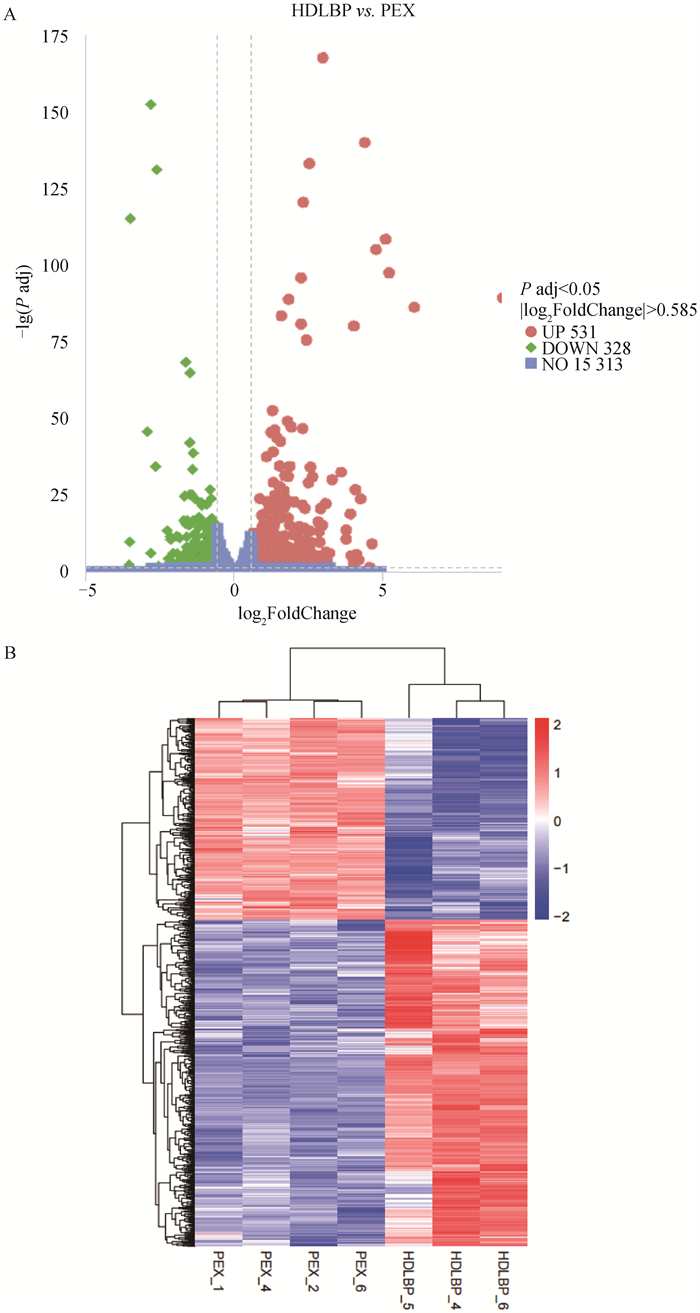
Table 2
The partial up- and down-regulated differentially expressed genes (DEGs)"
| 基因 Gene | 变化倍数的对数 log2(fold change) | Padj | |
| 上调 Up-regulated | CP | 2.97 | 2.38×10-168 |
| TNIP3 | 4.39 | 1.00×10-140 | |
| LOC106046725 | 2.52 | 7.01×10-134 | |
| LOC106046905 | 2.30 | 2.85×10-121 | |
| NOXO1 | 5.09 | 3.23×10-109 | |
| SLCO4C1 | 4.76 | 8.57×10-6 | |
| STEAP4 | 5.20 | 3.21×10-98 | |
| CHN2 | 2.24 | 1.44×10-96 | |
| SLC13A5 | 9.04 | 3.98×10-90 | |
| 下调 Down-regulated | DPYS | -2.84 | 3.24×10-153 |
| MAP3K7CL | -2.62 | 6.42×10-132 | |
| LOC106030908 | -3.52 | 6.02×10-116 | |
| PCK1 | -1.65 | 4.90×10-69 | |
| HRG | -1.51 | 1.52×10-65 | |
| TPH2 | -2.96 | 2.26×10-46 | |
| LOC106034448 | -1.52 | 6.91×10-43 | |
| GPR1 | -1.39 | 1.94×10-39 | |
| LOC106048076 | -2.67 | 4.63×10-35 | |
| CRACR2A | -1.43 | 4.83×10-34 |

Fig. 7
GO functional enrichment analysis of the differentially expressed genes(DEGs) Histogram of GO enrichment analysis. The X-axis of the graph denotes GO term, and Y-axis denotes the significance level of GO term enrichment, which is expressed as-lg(P adj). The different colors indicating different functional classifications"
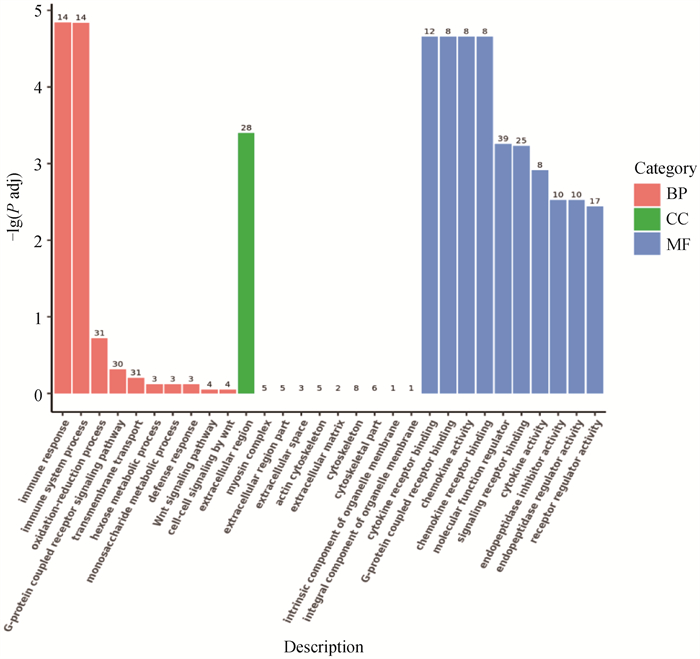

Fig. 8
The KEGG pathway enrichment analysis of the differentially expressed genes (DEGs) A. The up-regulated DEGs; B. The down-regulated DEGs. The X-axis indicates the ratio of the number of DEGs annotated to the KEGG pathway to the total number of the DEGs, the Y-axis indicates the KEGG pathways, the sizes of the points represent the number of genes annotated to the KEGG pathway, and the colors from red to purple represent the significant level of enrichment"

| 1 |
刘同君, 赵盼, 赵敏孟, 等. 内质网应激标记基因Grp78参与鹅肥肝免疫/炎症状态的调控[J]. 畜牧兽医学报, 2019, 50 (4): 727- 737.
doi: 10.11843/j.issn.0366-6964.2019.04.006 |
|
LIU T Q , ZHANG P , ZHAO M M , et al. Endoplasmic reticulum stress marker gene Grp78 Is involved in regulation of immune/inflammatory state of goose fatty liver[J]. Acta Veterinaria et Zootechnica Sinica, 2019, 50 (4): 727- 737.
doi: 10.11843/j.issn.0366-6964.2019.04.006 |
|
| 2 |
WEI R X , NING R , HAN C C , et al. Lipidomics analysis reveals new insights into the goose fatty liver formation[J]. Poult Sci, 2023, 102 (3): 102428.
doi: 10.1016/j.psj.2022.102428 |
| 3 |
LIU L , WANG Q , WANG Q Q , et al. Role of miR29c in goose fatty liver is mediated by its target genes that are involved in energy homeostasis and cell growth[J]. BMC Vet Res, 2018, 14 (1): 325.
doi: 10.1186/s12917-018-1653-3 |
| 4 | 柳序, 刘耀文, 匡佑华, 等. 鹅肥肝的形成及主要影响因素的研究进展[J]. 经济动物学报, 2019, 23 (4): 234- 239. |
| LIU X , LIU Y W , KUANG Y H , et al. Advances on formation and main influencing factors of foie Gras[J]. Journal of Economic Animal, 2019, 23 (4): 234- 239. | |
| 5 |
GENG T Y , YANG B , LI F Y , et al. Identification of protective components that prevent the exacerbation of goose fatty liver: Characterization, expression and regulation of adiponectin receptors[J]. Comp Biochem Physiol B Biochem Mol Biol, 2016, 194-195, 32- 38.
doi: 10.1016/j.cbpb.2016.01.006 |
| 6 |
XU A M , WANG Y , KESHAW H , et al. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice[J]. J Clin Invest, 2003, 112 (1): 91- 100.
doi: 10.1172/JCI200317797 |
| 7 |
KASER S , MOSCHEN A , CAYON A , et al. Adiponectin and its receptors in non-alcoholic steatohepatitis[J]. Gut, 2005, 54 (1): 117- 121.
doi: 10.1136/gut.2003.037010 |
| 8 |
STOWE D F , CAMARA A K S . Mitochondrial reactive oxygen species production in excitable cells: modulators of mitochondrial and cell function[J]. Antioxid Redox Signal, 2009, 11 (6): 1373- 1414.
doi: 10.1089/ars.2008.2331 |
| 9 |
陈翠英, 邵明亮, 杨莉, 等. 乌丹降脂方对脂肪肝大鼠脂肪肝细胞氧化损伤调节作用研究[J]. 现代中西医结合杂志, 2016, 25 (20): 2180-2182, 2198.
doi: 10.3969/j.issn.1008-8849.2016.20.005 |
|
CHEN C Y , SHAO M L , YANG L , et al. Study on the regulation of Wudan Jiangzhi decoction on peroxidation damage in liver cells of the rats with liver fat[J]. Modern Journal of Integrated Traditional Chinese and Western Medicine, 2016, 25 (20): 2180-2182, 2198.
doi: 10.3969/j.issn.1008-8849.2016.20.005 |
|
| 10 |
PENG K Y , WATT M J , RENSEN S , et al. Mitochondrial dysfunction-related lipid changes occur in nonalcoholic fatty liver disease progression[J]. J Lipid Res, 2018, 59 (10): 1977- 1986.
doi: 10.1194/jlr.M085613 |
| 11 | 沈文婷, 陈明, 许诣, 等. PDTC调节线粒体功能障碍与Klotho蛋白表达对大鼠脓毒症合并急性肾损伤的影响[J]. 广西医科大学学报, 2021, 38 (11): 2097- 2103. |
| SHEN W T , CHEN M , XU Y , et al. The effects of PDTC regulating mitochondrial dysfunction and Klotho protein expression on acute kidney injury in rat with sepsis[J]. Journal of Guangxi Medical University, 2021, 38 (11): 2097- 2103. | |
| 12 | 郭燕, 徐爽, 王亭. PKD1抑制剂CID755673通过诱导线粒体功能障碍加重急性肾损伤[J]. 临床与实验病理学杂志, 2023, 39 (2): 206-211, 215. |
| GUO Y , XU S , WANG T . PKD1 inhibitor CID755673 aggravates acute kidney injury by inducing mitochondrial dysfunction[J]. Chinese Journal of Clinical and Experimental Pathology, 2023, 39 (2): 206-211, 215. | |
| 13 | 朱潇旭, 段小花, 李瑞霞, 等. 线粒体功能障碍与非酒精性脂肪肝发病关系的研究进展[J]. 山东医药, 2018, 58 (29): 108- 111. |
| ZHU X X , DUAN X H , LI R X , et al. Research progress on the relationship between mitochondrial dysfunction and nonalcoholic fatty liver disease[J]. Shandong Medicine, 2018, 58 (29): 108- 111. | |
| 14 |
SUN Q Y , DAI E P , CHEN M , et al. Glucose-induced enhanced anti-oxidant activity inhibits apoptosis in goose fatty liver[J]. J Anim Sci, 2023, 101, skad059.
doi: 10.1093/jas/skad059 |
| 15 | FEICHT J , JANSEN R P . The high-density lipoprotein binding protein HDLBP is an unusual RNA-binding protein with multiple roles in cancer and disease[J]. RNA Biol, 2024, 21 (1): 1- 10. |
| 16 |
CHENG M H K , JANSEN R P . A jack of all trades: the RNA-binding protein vigilin[J]. Wiley Interdiscip Rev RNA, 2017, 8 (6): e1448.
doi: 10.1002/wrna.1448 |
| 17 |
DOMÍNGUEZ-PÉREZ M , SIMONI-NIEVES A , ROSALES P , et al. Cholesterol burden in the liver induces mitochondrial dynamic changes and resistance to apoptosis[J]. J Cell Physiol, 2019, 234 (5): 7213- 7223.
doi: 10.1002/jcp.27474 |
| 18 | ELUSTONDO P , MARTIN L A , KARTEN B . Mitochondrial cholesterol import[J]. Biochim Biophys Acta Mol Cell Biol Lipids, 2017, 1862 (1): 90- 101. |
| 19 |
DUAN Y J , GONG K , XU S W , et al. Regulation of cholesterol homeostasis in health and diseases: from mechanisms to targeted therapeutics[J]. Signal Transduct Target Ther, 2022, 7 (1): 265.
doi: 10.1038/s41392-022-01125-5 |
| 20 | 洪胜辉, 张军, 张蕊, 等. 鹅原代肝细胞的简易、高纯分离及培养[J]. 江苏农业科学, 2012, 40 (4): 56- 58. |
| HONG S H , ZHANG J , ZHANG R , et al. Simple, high-purity isolation and culture of goose primary hepatocytes[J]. Jiangsu Agricultural Sciences, 2012, 40 (4): 56- 58. | |
| 21 |
ZENG S , WU F , CHEN M Y , et al. Inhibition of fatty acid translocase (FAT/CD36) palmitoylation enhances hepatic fatty acid β-oxidation by increasing its localization to mitochondria and interaction with long-chain acyl-CoA synthetase 1[J]. Antioxid Redox Signal, 2022, 36 (16-18): 1081- 1100.
doi: 10.1089/ars.2021.0157 |
| 22 |
MOBIN M B , GERSTBERGER S , TEUPSER D , et al. The RNA-binding protein vigilin regulates VLDL secretion through modulation of ApoB mRNA translation[J]. Nat Commun, 2016, 7 (1): 12848.
doi: 10.1038/ncomms12848 |
| 23 |
GUO X Y , YIN X Z , LIU Z J , et al. Non-alcoholic fatty liver disease (NAFLD) pathogenesis and natural products for prevention and treatment[J]. Int J Mol Sci, 2022, 23 (24): 15489.
doi: 10.3390/ijms232415489 |
| 24 | 周小艺, 邢娅, 龚道清, 等. 线粒体和鹅肥肝形成的关系[J]. 动物营养学报, 2023, 35 (2): 699- 707. |
| ZHOU X Y , XING Y , GONG D Q , et al. Relationship between mitochondria and goose fatty liver formation[J]. Chinese Journal of Animal Nutrition, 2023, 35 (2): 699- 707. | |
| 25 | DRAKE J C , WILSON R J , LAKER R C , et al. Mitochondria-localized AMPK responds to local energetics and contributes to exercise and energetic stress-induced mitophagy[J]. Proc Natl Acad Sci U S A, 2021, 118 (37): e2025932118. |
| 26 | ZHANG Q , DIDONATO J A , KARIN M , et al. BCL3 encodes a nuclear protein which can alter the subcellular location of NF-κB proteins[J]. Mol Cell Biol, 1994, 14 (6): 3915- 3926. |
| 27 | 谢业成, 郭仪琳, 李雪露, 等. BCL3转录共激活因子的亚细胞定位研究[J]. 中国细胞生物学学报, 2021, 43 (8): 1574- 1580. |
| XIE Y C , GUO Y L , LI X L , et al. Investigation on subcellular localization of BCL3 transcription coactivator[J]. Chinese Journal of Cell Biology, 2021, 43 (8): 1574- 1580. | |
| 28 | VON ECKARDSTEIN A , NOFER J R , ASSMANN G . High density lipoproteins and arteriosclerosis.Role of cholesterol efflux and reverse cholesterol transport[J]. Arterioscler Thromb Vasc Biol, 2001, 21 (1): 13- 27. |
| 29 | 郑吉春, 王韫芳, 裴雪涛. 胆汁酸代谢对肝细胞功能的影响[J]. 肝脏, 2007, 12 (4): 308- 310. |
| ZHENG J C , WANG Y F , PEI X T . Effects of bile acids metabolism on hepatocytes function[J]. Chinese Hepatology, 2007, 12 (4): 308- 310. | |
| 30 | MARTIN L A , KENNEDY B E , KARTEN B . Mitochondrial cholesterol: mechanisms of import and effects on mitochondrial function[J]. J Bioenerg Biomembr, 2016, 48 (2): 137- 151. |
| 31 | GOICOECHEA L , DE LA ROSA L C , TORRES S , et al. Mitochondrial cholesterol: Metabolism and impact on redox biology and disease[J]. Redox Biol, 2023, 61, 102643. |
| 32 | AHMAD S , YTTERBERG A J , THULASINGAM M , et al. Phosphorylation of leukotriene C4 synthase at serine 36 impairs catalytic activity[J]. J Biol Chem, 2016, 291 (35): 18410- 18418. |
| 33 | WANG Y S , WANG J , ZHENG W J , et al. Identification of an IL-1 receptor mutation driving autoinflammation directs IL-1-targeted drug design[J]. Immunity, 2023, 56 (7): 1485- 1501.e7. |
| 34 | TANG W H , WANG W M , ZHANG Y X , et al. Tumour necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced chemokine release in both TRAIL-resistant and TRAIL-sensitive cells via nuclear factor kappa B[J]. FEBS J, 2009, 276 (2): 581- 593. |
| 35 | HOLMDAHL R , SAREILA O , OLSSON L M , et al. Ncf1 polymorphism reveals oxidative regulation of autoimmune chronic inflammation[J]. Immunol Rev, 2016, 269 (1): 228- 247. |
| 36 | TOUAT-HAMICI Z , WEIDMANN H , BLUM Y , et al. Role of lipid phosphate phosphatase 3 in human aortic endothelial cell function[J]. Cardiovasc Res, 2016, 112 (3): 702- 713. |
| 37 | CUI Y N , ZHANG P P , LIANG X , et al. Association of KDR mutation with better clinical outcomes in pan-cancer for immune checkpoint inhibitors[J]. Am J Cancer Res, 2022, 12 (4): 1766- 1783. |
| 38 | THORENOOR N , ZHANG X S , UMSTEAD T M , et al. Differential effects of innate immune variants of surfactant protein-A1 (SFTPA1) and SP-A2 (SFTPA2) in airway function after Klebsiella pneumoniae infection and sex differences[J]. Respir Res, 2018, 19 (1): 23. |
| [1] | Shuo YANG, Min HUO, Zixuan SU, Yuxiang SHI. Research Progress on the Impact of Mitochondrial Quality Control on Oxidative Stress in Livestock and Poultry [J]. Acta Veterinaria et Zootechnica Sinica, 2024, 55(9): 3769-3776. |
| [2] | HUANG Hongyan, ZHANG Liyun, HUANG Zhirong, WU Zhongping, ZHANG Xumeng, OUYANG Hongjia, CHEN Junpeng, LIN Zhenping, TIAN Yunbo, LI Xiujin, HUANG Yunmao. The Study on Population Genetic Diversity and Genome-wide Association Study of Body Weight and Size Traits for Lion-head Geese [J]. Acta Veterinaria et Zootechnica Sinica, 2024, 55(9): 3914-3924. |
| [3] | WANG Yi, GONG Jianfei, HENG Nuo, HU Yingfan, WANG Rui, WANG Huan, ZHU Ni, HE Wei, HU Zhihui, HAO Haisheng, ZHU Huabin, ZHAO Shanjiang. Melatonin Alleviates Palmitic Acid-induced Damage in Bovine Endometrial Epithelial Cells by Improving Mitochondrial Dynamics [J]. Acta Veterinaria et Zootechnica Sinica, 2024, 55(9): 3978-3987. |
| [4] | LIU Xinman, ZHOU Hongyuan, SANG Rui, GE Bingjie, YAN Kexin, WANG Wei, YU Minghong, LIU Xiaotong, QIU Qian, ZHANG Xuemei. Effect of Taraxasterol on Oxidative Stress in Liver Tissue of Broilers with AFB1 Induced Liver Injury [J]. Acta Veterinaria et Zootechnica Sinica, 2024, 55(9): 4141-4152. |
| [5] | Jingxuan WANG, Lizhi DAI, Zhenyu WANG, Ying LIU, Tong YU, Min YAN, Ruilong WANG, Jianhua XIAO. Study on the Characteristics of Liver Energy Metabolism during the Induction of Insulin Resistance by High Fat Diet [J]. Acta Veterinaria et Zootechnica Sinica, 2024, 55(9): 4172-4185. |
| [6] | Yan WANG, Yadong GAO, Chenghui JIANG, Qiaoying ZENG. Isolation and Pathogenicity of a Goose Derived Fowl Adenovirus Type 4 [J]. Acta Veterinaria et Zootechnica Sinica, 2024, 55(9): 4232-4240. |
| [7] | Ya’nan LI, Tianwen MA, Yuhui MA, Chengwei WEI. Bilobalide Regulates Mitochondrial Biogenesis Mediated by AMPK-SIRT3 Positive Feedback Loop and Improves Inflammatory Damage of ATDC5 Chondrocytes [J]. Acta Veterinaria et Zootechnica Sinica, 2024, 55(8): 3714-3724. |
| [8] | Yaxuan MENG, Yan LIU, Jing WANG, Guoshun CHEN, Tao FENG. Research Progress in the Effect of Oxidative Stress on Ovarian Function in Female Livestock [J]. Acta Veterinaria et Zootechnica Sinica, 2024, 55(7): 2825-2835. |
| [9] | Yuanyuan LI, Tianyu WANG, Meng LI, Wenhui ZHANG, Yinghui WANG, Tianrui ZHAO, Haojie LI, Yangfei ZHAO, Jinming WANG. Selenomethionine, through PINK1/Parkin-mediated Mitochondrial Autophagy, Alleviates Fluoride-induced Depressive-like Behavior [J]. Acta Veterinaria et Zootechnica Sinica, 2024, 55(7): 3213-3224. |
| [10] | Qianling CHEN, Yuzhu SHA, Xiu LIU, Pengyang SHAO, Fanxiong WANG, Xiaowei CHEN, Wenxin YANG, Zhuanhui XIE, Min GAO, Wei HUANG. Research Progress on the Interaction between Gut Microbiota and Mitochondria Regulating Animal Fat Deposition [J]. Acta Veterinaria et Zootechnica Sinica, 2024, 55(6): 2293-2303. |
| [11] | CHEN Zhe, QU Xiaolu, GUO Binbin, SUN Xuefeng, YAN Leyan. Study on Candidate Genes for Green Light Affecting Early Development of Goose Embryo Heart Based on Transcriptome Sequencing [J]. Acta Veterinaria et Zootechnica Sinica, 2024, 55(5): 1978-1988. |
| [12] | LI Feifei, ZHANG Chenmiao, TONG Jinjin, JIANG Linshu. Research Progress on the Mechanism of Mitochondrial Autophagy Regulating the Activity of NLRP3 Inflammatory Corpuscles to Improve Animal Health [J]. Acta Veterinaria et Zootechnica Sinica, 2024, 55(4): 1446-1455. |
| [13] | ZHANG Xinting, QIU Wenyue, PANG Xiaoyue, SU Yiman, YE Jiali, HUANG Jianjia, ZHOU Shuilian, TANG Zhaoxin, WANG Rongmei, SU Rongsheng. Effect of Asiatic Acid Alleviating Myocardial Injury Caused by Lipopolysaccharide through Inhibiting Oxidative Stress and Ferroptosis in Broilers [J]. Acta Veterinaria et Zootechnica Sinica, 2024, 55(4): 1787-1799. |
| [14] | JIANG Lijun, ZONG Yunhe, LI Yunlei, CHEN Jilan, GENG Zhaoyu, SUN Yanyan, JIN Sihua. Research Progress of Antioxidant Application in Poultry Semen Storage [J]. Acta Veterinaria et Zootechnica Sinica, 2024, 55(3): 913-923. |
| [15] | SHEN Wenjuan, YANG Zhuo, ZHANG Xinrui, FU Yu, TAO Jinzhong. Research Progress of Microorganisms and Reproductive and Related Diseases in Dairy Cows Reproductive Tract [J]. Acta Veterinaria et Zootechnica Sinica, 2024, 55(3): 924-932. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||