





Acta Veterinaria et Zootechnica Sinica ›› 2024, Vol. 55 ›› Issue (7): 3132-3142.doi: 10.11843/j.issn.0366-6964.2024.07.031
• Basic Veterinary Medicine • Previous Articles Next Articles
Yusheng JIA( ), Yilin LI, Lulu MA, Ming LIAO, Manman DAI*(
), Yilin LI, Lulu MA, Ming LIAO, Manman DAI*( )
)
Received:2023-10-19
Online:2024-07-23
Published:2024-07-24
Contact:
Manman DAI
E-mail:jiayusheng0922@163.com;daimanman1229@scau.edu.cn
CLC Number:
Yusheng JIA, Yilin LI, Lulu MA, Ming LIAO, Manman DAI. Structure and Mechanism of ALV-J Epitope Presented by MHC Class Ⅰ Molecule BF2*0201[J]. Acta Veterinaria et Zootechnica Sinica, 2024, 55(7): 3132-3142.

Fig. 1
Molecular sieve purification of pBF2*0201 protein Fig. A, B and C are the results of in vitro complexation of three peptides with BF2*0201 and Chβ2m and purification using an S200 molecular sieve chromatography column(SuperdexTTM 200 Increase 10/300 GL); Fig. D and E are the result of in vitro complexation of peptide SALQAFREV and FVDFANRLI with BF2*0201 and Chβ2m and purification using a B200 molecular sieve chromatography column(HiLoadTM16/600 SuperdexTM 200 pg)"

Table 1
Data collection and refinement statistics"
| 参数 Parameter | BF2*0201-SV9的数值 Value in BF2*0201-SV9 |
| 数据处理 Data processing | |
| Space group | P1211 |
| Cell parameters (Å) | 86.614 115.584 94.107 90 111.158 90 |
| Resolution range (Å) | 43.06~1.72 (1.781~1.72) |
| Total reflections | 354 688 (34 360) |
| Unique reflections | 180 646 (17 706) |
| Completeness (%) | 98.68 (97.06) |
| Rmerge | 0.042 48 (0.314 4) |
| 重修 Refinement | |
| Rwork | 0.183 1 (0.254 4) |
| Rfree | 0.215 0 (0.281 8) |
| RMSD | |
| Bonds (Å) | 0.010 |
| Angles (°) | 1.61 |
| Average B factor | 21.90 |
| 拉氏图质量 Ramachandran plot quality | |
| Most favored (%) | 98.93 |
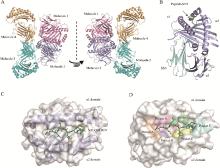
Fig. 2
Overall structure of BF2*0201-SV9 and structure of peptide-binding groove A. The crystal cell of pBF2*0201, different colors represent different crystal molecules. Molelule2 was the molecule investigated subsequently in this experiment, which was colored by mauve; B. Overall structure of the pBF2*0201 molecule, with α1, α2 and α3 representing the structural domains of the MHC, and the short peptide indicated by the arrowheads is the peptide that is delivered by the MHC Ⅰ; C. Structure of the peptide-binding groove of pBF2*0201, which is the formation of eight β-sheet layers and two α-helical structures; D. Correspondence between peptides and pockets"
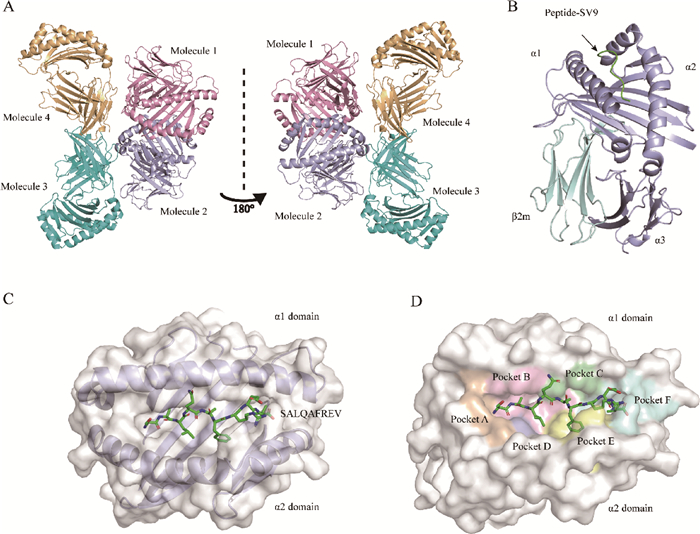
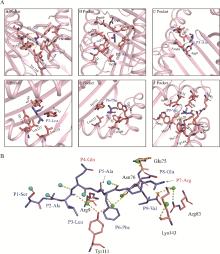
Fig. 3
Forces of SV9 with BF2*0201 pocket amino acids and water molecules A. Amino acid composition of the pocket for BF2*0201 and interaction forces with the peptide. Hydrogen bonds were labeled in yellow; B. Hydrogen bonding forces marked in yellow between water molecules in the antigen-binding groove with antigenic polypeptides and pocket amino acids. The green spheres represent water molecules connecting the antigenic polypeptide with the pocket amino acid, and the light blue spheres represent water molecules interacting only with the antigenic polypeptide"

| 1 |
MO G D , FU H L , HU B W , et al.SOCS3 Promotes ALV-J virus replication via inhibiting JAK2/STAT3 phosphorylation during infection[J].Front Cell Infect Microbiol,2021,11,748795.
doi: 10.3389/fcimb.2021.748795 |
| 2 |
CHEN W G , CHEN S , NIE Y , et al.Synergistic immunosuppression of avian leukosis virus subgroup j and infectious bursal disease virus is responsible for enhanced pathogenicity[J].Viruses,2022,14(10):2312.
doi: 10.3390/v14102312 |
| 3 |
LI H J , WANG P K , LIN L L , et al.The emergence of the infection of subgroup J avian leucosis virus escalated the tumour incidence in commercial yellow chickens in Southern China in recent years[J].Transbound Emerg Dis,2019,66(1):312-316.
doi: 10.1111/tbed.13023 |
| 4 |
PAYNE L N , NAIR V .The long view: 40 years of avian leukosis research[J].Avian Pathol,2012,41(1):11-19.
doi: 10.1080/03079457.2011.646237 |
| 5 | 兰玲, 王涛.新疆养禽场禽白血病流行病学调查[J].新疆畜牧业,2017,32(11):52, 39. |
| LAN L , WANG T .Epidemiological survey of avian leukosis in poultry farms in Xinjiang[J].Animal Husbandry in Xinjiang,2017,32(11):52, 39. | |
| 6 | 俞燕, 徐步, 周生, 等. 地方品种鸡禽白血病流行病学调查[Z]. 南宁: 20181. |
| YU Y, XU B, ZHOU S, et al. Epidemiological survey of avian leukosis in local breeds of chicken[Z]. Nanning: 20181. (in Chinese) | |
| 7 | 葛成, 焦贺静, 张海龙, 等.河北省蛋种鸡ALV携带率及亚群检测[J].中国家禽,2019,41(21):66-68. |
| GE C , JlAO H J , ZHANG H L , et al.Carrying rate and subgroup detection of AlV in layer breeders in Hebei Province[J].China Poultry,2019,41(21):66-68. | |
| 8 |
ZHOU D F , XUE J W , ZHANG Y , et al.Outbreak of myelocytomatosis caused by mutational avian leukosis virus subgroup J in China, 2018[J].Transbound Emerg Dis,2019,66(2):622-626.
doi: 10.1111/tbed.13096 |
| 9 |
DAI M M , LI S B , SHI K Y , et al.Systematic identification of host immune key factors influencing viral infection in PBL of ALV-J infected SPF chicken[J].Viruses,2020,12(1):114.
doi: 10.3390/v12010114 |
| 10 |
JONGSMA M L M , GUARDA G , SPAAPEN R M .The regulatory network behind MHC class I expression[J].Mol Immunol,2019,113,16-21.
doi: 10.1016/j.molimm.2017.12.005 |
| 11 |
KOCH M , CAMP S , COLLEN T , et al.Structures of an MHC class I molecule from B21 chickens illustrate promiscuous peptide binding[J].Immunity,2007,27(6):885-899.
doi: 10.1016/j.immuni.2007.11.007 |
| 12 |
WALLNY H J , AVILA D , HUNT L G , et al.Peptide motifs of the single dominantly expressed class I molecule explain the striking MHC-determined response to Rous sarcoma virus in chickens[J].Proc Natl Acad Sci U S A,2006,103(5):1434-1439.
doi: 10.1073/pnas.0507386103 |
| 13 |
DA SILVA A P , GALLARDO R A .The chicken MHC: insights into genetic resistance, immunity, and inflammation following infectious bronchitis virus infections[J].Vaccines (Basel),2020,8(4):637.
doi: 10.3390/vaccines8040637 |
| 14 |
BACON L D , WITTER R L , CRITTENDEN L B , et al.B-haplotype influence on Marek's disease, rous sarcoma, and lymphoid leukosis virus-induced tumors in chickens[J].Poult Sci,1981,60(6):1132-1139.
doi: 10.3382/ps.0601132 |
| 15 |
ZHANG J H , CHEN Y , QI J X , et al.Narrow groove and restricted anchors of MHC class I molecule BF2*0401 plus peptide transporter restriction can explain disease susceptibility of B4 chickens[J].J Immunol,2012,189(9):4478-4487.
doi: 10.4049/jimmunol.1200885 |
| 16 | JENSEN L H .Refinement and reliability of macromolecular models based on X-ray diffraction data[J].Methods Enzymol,1997,277,353-366. |
| 17 |
CHAPPELL P E , MEZIANE E K , HARRISON M , et al.Expression levels of MHC class I molecules are inversely correlated with promiscuity of peptide binding[J].Elife,2015,4,e05345.
doi: 10.7554/eLife.05345 |
| 18 | LEBEDEV A A , VAGIN A A , MURSHUDOV G N .Model preparation in MOLREP and examples of model improvement using X-ray data[J].Acta Crystallogr D Biol Crystallogr,2008,64(Pt 1):33-39. |
| 19 | EMSLEY P , COWTAN K .Coot: model-building tools for molecular graphics[J].Acta Crystallogr D Biol Crystallogr,2004,60(Pt 12 Pt 1):2126-2132. |
| 20 | MURSHUDOV G N , VAGIN A A , DODSON E J .Refinement of macromolecular structures by the maximum-likelihood method[J].Acta Crystallogr D Biol Crystallogr,1997,53(Pt 3):240-255. |
| 21 | ADAMS P D , GROSSE-KUNSTLEVE R W , HUNG L W , et al.PHENIX: building new software for automated crystallographic structure determination[J].Acta Crystallogr D Biol Crystallogr,2002,58(Pt 11):1948-1954. |
| 22 | AGIRRE J , ATANASOVA M , BAGDONAS H , et al.The CCP4 suite: integrative software for macromolecular crystallography[J].Acta Crystallogr D Struct Biol,2023,79(Pt 6):449-461. |
| 23 | 肖进. 鸡MHCI分子呈递病毒多肽的晶体结构及功能研究[D]. 北京: 中国农业大学, 2017. |
| XIAO J. Study on the crystal structure and function of chicken MHCI presenting viral peptide[D]. Beijing: China Agricultural University, 2017. (in Chinese) | |
| 24 | 吴亚楠. 四种低等脊椎动物pMHC I复合体或β2m分子的晶体结构研究[D]. 北京: 中国农业大学, 2017. |
| WU Y N. Study on the crystal structures of pMHCI complexes or β2m molecules in four lower vertebrates[D]. Beijing: China Agricultural University, 2017. (in Chinese) | |
| 25 | WIECZOREK M , ABUALROUS E T , STICHT J , et al.Major histocompatibility complex (MHC) class I and MHC class Ⅱ proteins: conformational plasticity in antigen presentation[J].Front Immunol,2017,8,292. |
| 26 | LIU Y J , CHEN R , LIANG R Y , et al.The combination of CD8αα and peptide-MHC-I in a face-to-face mode promotes chicken γδT cells response[J].Front Immunol,2020,11,605085. |
| 27 | WU Y A , ZHANG N Z , WEI X H , et al.The structure of a peptide-loaded shark MHC class I molecule reveals features of the binding between β2-microglobulin and H chain conserved in evolution[J].J Immunol,2021,207(1):308-321. |
| 28 | SILVER M L , PARKER K C , WILEY D C .Reconstitution by MHC-restricted peptides of HLA-A2 heavy chain with β2-microglobulin, in vitro[J].Nature,1991,350(6319):619-622. |
| 29 | MITAKSOV V , FREMONT D H .Structural definition of the H-2Kd peptide-binding motif[J].J Biol Chem,2006,281(15):10618-10625. |
| 30 | HALABI S , GHOSH M , STEVANOVI? S , et al.The dominantly expressed class Ⅱ molecule from a resistant MHC haplotype presents only a few Marek's disease virus peptides by using an unprecedented binding motif[J].PLoS Biol,2021,19(4):e3001057. |
| 31 | ROCK K L , REITS E , NEEFJES J .Present yourself!By MHC class I and MHC class Ⅱ molecules[J].Trends Immunol,2016,37(11):724-737. |
| 32 | SAPER M A , BJORKMAN P J , WILEY D C .Refined structure of the human histocompatibility antigen HLA-A2 at 2. 6 Å resolution[J].J Mol Biol,1991,219(2):277-319. |
| 33 | ZHANG L , LI Z L , TANG Z C , et al.Efficient identification of tembusu virus CTL Epitopes in inbred HBW/B4 ducks using a novel MHC class I-restricted epitope screening scheme[J].J Immunol,2022,209(1):145-156. |
| 34 | 樊淑华. 猪MHCI晶体结构及结合IAV和PRRSV表位特性的研究[D]. 北京: 中国农业大学, 2016. |
| FAN S H. Structural analyses of swine MHC class I complexes and their binding epitopes of IAV and PRRSV[D]. Beijing: China Agricultural University, 2016. (in Chinese) | |
| 35 | GAO Y L , QIN L T , PAN W , et al.Avian leukosis virus subgroup J in layer chickens, China[J].Emerg Infect Dis,2010,16(10):1637-1638. |
| 36 | LI Y , FU J Y , CUI S , et al.Gp85 genetic diversity of avian leukosis virus subgroup J among different individual chickens from a native flock[J].Poult Sci,2017,96(5):1100-1107. |
| 37 | LIN W C , LI X J , DAI Z K , et al.Molecular epidemiology of J-subgroup avian leukosis virus isolated from meat-type chickens in southern China between 2013 and 2014[J].Arch Virol,2016,161(11):3039-3046. |
| 38 | YE F , WANG Y , HE Q J , et al.Exosomes transmit viral genetic information and immune signals may cause immunosuppression and immune tolerance in ALV-J infected HD11 cells[J].Int J Biol Sci,2020,16(6):904-920. |
| 39 | ZINKERNAGEL R M , DOHERTY P C .Restriction of in vitro T cell-mediated cytotoxicity in lymphocytic choriomeningitis within a syngeneic or semiallogeneic system[J].Nature,1974,248(5450):701-702. |
| 40 | XIAO J , XIANG W Z , ZHANG Y L , et al.An invariant arginine in common with MHC class Ⅱ allows extension at the C-terminal end of peptides bound to chicken MHC class I[J].J Immunol,2018,201(10):3084-3095. |
| 41 | LI X Y , ZHANG L J , LIU Y J , et al.Structures of the MHC-I molecule BF2*1501 disclose the preferred presentation of an H5N1 virus-derived epitope[J].J Biol Chem,2020,295(16):5292-5306. |
| 42 | NIVARTHI U K , GRAS S , KJER-NIELSEN L , et al.An extensive antigenic footprint underpins immunodominant TCR adaptability against a hypervariable viral determinant[J].J Immunol,2014,193(11):5402-5413. |
| 43 | HALABI S , KAUFMAN J .New vistas unfold: chicken MHC molecules reveal unexpected ways to present peptides to the immune system[J].Front Immunol,2022,13,886672. |
| 44 | JIN Y C , WANG W , YU M M , et al.Study on the contrast of the MHC-peptide interaction of B2/B21 haplotype and MHC-related virus resistance in chickens[J].Immun Inflamm Dis,2021,9(4):1670-1677. |
| 45 | DAI M M , XU C G , CHEN W S , et al.Progress on chicken T cell immunity to viruses[J].Cell Mol Life Sci,2019,76(14):2779-2788. |
| 46 | WEI X H , WANG S , LI Z L , et al.Peptidomes and structures illustrate two distinguishing mechanisms of alternating the peptide plasticity caused by swine MHC class I micropolymorphism[J].Front Immunol,2021,12,592447. |
| 47 | KHEIMAR A , KLINGER R , BERTZBACH L D , et al.A genetically engineered commercial chicken line is resistant to highly pathogenic avian leukosis virus subgroup J[J].Microorganisms,2021,9(5):1066. |
| 48 | FENG M , ZHANG N , XIE T T , et al.Chichen type Ⅲ interferon produced by silkworm bioreactor induces ISG expression and restricts ALV-J infection in vitro[J].Appl Microbiol Biotechnol,2019,103(20):8473-8483. |
| [1] | DONG Xinyi, LI Jinqun, CHEN Qinxi, LIAO Ming, CAO Weisheng. Establishment of Blood Nucleic Acid Screening Technology for Subgroup J Avian Leukosis Virus [J]. Acta Veterinaria et Zootechnica Sinica, 2024, 55(3): 1115-1126. |
| [2] | FENG Weimin, LIU Xiao, HUANG Teng. The Evasion Strategy against CTL Recognition by Herpesviruses of Domestic Animals: Interference with MHC Class Ⅰ Antigen Presentation Pathway [J]. Acta Veterinaria et Zootechnica Sinica, 2023, 54(6): 2241-2251. |
| [3] | LIU Junhong, ZHAO Yue, LI Hongmei, WANG Ying, WANG Moyu, CHENG Ziqiang, QIU Jianhua, HOU Qiuling, GUO Huijun. Comparison of Tissue Hyperplasia Lesions and Cytokines' Expressions Induced by Two Different Avian Leukosis Virus Subgroup J Strains [J]. Acta Veterinaria et Zootechnica Sinica, 2022, 53(6): 1895-1904. |
| [4] | CHEN Sheng, LIAO Zhihong, XIE Zi, CHEN Feng, XIE Qingmei, SHU Wei. GADD45β Suppresses the Replication of Avian Leukosis Virus Subgroup J in DF-1 Cells [J]. Acta Veterinaria et Zootechnica Sinica, 2021, 52(6): 1662-1669. |
| [5] | ZHANG Huiyong, WU Jingsheng, YANG Jianbo, YU Yan, XUE Qian, YIN Jianmei, ZHU Yunfen, ZHU Jing, SU Yijun, LI Guohui, HAN Wei. Application of RNA-Seq Technology for Screening the Differentially Expressed Pathogenic Genes in Wenshang Barred Chicken Liver Infected by Subgroup J Avian Leukosis Virus [J]. Acta Veterinaria et Zootechnica Sinica, 2020, 51(8): 2003-2011. |
| [6] | GAO Yan-ni,GAO Qi,LI Xiao-fei,YUN Bing-ling,QI Xiao-le,WANG Yong-qiang,LIU Chang-jun,CUI Hong-yu,ZHANG Yan-ping,GAO Hong-lei,WANG Xiao-mei,GAO Yu-long. The Mutations of 3′-U3 Region Make No Difference to ALV’s Replication Capacity in vitro [J]. ACTA VETERINARIA ET ZOOTECHNICA SINICA, 2015, 46(4): 608-614. |
| [7] | WANG Guang-wen,Lü Lin,WANG Chao,ZHANG Zhen-dong,CHENG Zi-long,QU Ya-jin,LIU Si-dang. Analysis of Astragalan and Thiamphenicol Affecting ALV-J Pathogenicity on SPF Chicken [J]. ACTA VETERINARIA ET ZOOTECHNICA SINICA, 2015, 46(10): 1858-1866. |
| [8] | QIN Si-hai,LIU Li-tao,YUE Rui-chao,LI Ning,QU Ya-jin,WANG Guang-wen,HU Dong-fang,LIU Si-dang. Study on Comprehensive Diagnosis and Molecular Characterization of Subgroup J Avian Leukosis Virus from Silkies [J]. ACTA VETERINARIA ET ZOOTECHNICA SINICA, 2014, 45(3): 458-462. |
| [9] | LI Ming, ZHANG Jun-hua, SUN Zhao-jun, CHEN Wei-min. The Expression of MHC in Uterus and Placenta of Pregnant Rat and Its Regulation by TGF-β1 [J]. ACTA VETERINARIA ET ZOOTECHNICA SINICA, 2013, 44(5): 802-810. |
| [10] | LI De-qing, ZHAO Peng, WANG Xin, WANG Xiao-fei, CUI Zhi-zhong. Comparison of the Pathogenicity of ALV-J Related Acute Fibrosarcoma ExtractInoculated on Embryos and Chicks [J]. ACTA VETERINARIA ET ZOOTECHNICA SINICA, 2013, 44(2): 250-256. |
| [11] | WANG Bei, LIU Fang, WANG Han-qing, LI Ning, WEI Jian-zhong, LIU Guang-qing, WANG Gui-jun. Construction of Infectious Clone of Avian Leucosis Virus Subgroup J AH-J11 Strain [J]. ACTA VETERINARIA ET ZOOTECHNICA SINICA, 2013, 44(12): 1976-1981. |
| [12] | ZHU Xiao-qing, LI Xiao-zhen, QIAO Hai-bo, JIA Shu-hong, ZHANG Dong-sheng, GU Xin-li. Effects of Polysaccharide Extracted from 11 Kinds of Traditional Chinese Medicines on ND Antibody Level and IFN-γ, IL-4 Contents in Different MHC B-LβⅡ Genotype Chickens [J]. ACTA VETERINARIA ET ZOOTECHNICA SINICA, 2013, 44(10): 1685-1692. |
| [13] | LIU Zi-Zhan, WANG Zai-Gui, LI Kui, GUO Ya-Fen, WANG Ai-De, ZHANG Yu-Qiong, YANG Shu-Lin. Cloning and Bioinformatics Analysis of Swine Leukocyte Antigens ClassⅡ Genes in Guangxi Bama Minipigs [J]. ACTA VETERINARIA ET ZOOTECHNICA SINICA, 2012, 43(9): 1353-1359. |
| [14] | JIANG Cheng-lan, XING Feng,XU Yun-hua, WANG Meng, LI Yan-ping. Cloning and Homology of the Functional Region of Porcine NMMHC-ⅡA Gene and Its Expression Pattern in Pigs [J]. ACTA VETERINARIA ET ZOOTECHNICA SINICA, 2012, 43(10): 1519-1524. |
| [15] | ZHAO Peng, CUI Zhi-zhong, MA Cheng-tai. Detection of Antigen P27 in Eggs and Its Correlation with the Avian Leukosis Virus Infection in Different Breeder or Commercial Chickens [J]. ACTA VETERINARIA ET ZOOTECHNICA SINICA, 2012, 43(10): 1618-1622. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||