





Acta Veterinaria et Zootechnica Sinica ›› 2025, Vol. 56 ›› Issue (11): 5864-5874.doi: 10.11843/j.issn.0366-6964.2025.11.042
• Basic Veterinary Medicine • Previous Articles Next Articles
CAO Qiuxia1,2( ), YAN Kexin2, CHENG Zhenkong2, BIAN Xianyu2, WANG Chuanhong2, LI Sufen2, ZHANG Xuehan2, FAN Baochao2, GUO Rongli2, YANG Shanshan2,*(
), YAN Kexin2, CHENG Zhenkong2, BIAN Xianyu2, WANG Chuanhong2, LI Sufen2, ZHANG Xuehan2, FAN Baochao2, GUO Rongli2, YANG Shanshan2,*( ), WANG Xiaodu1,*(
), WANG Xiaodu1,*( ), LI Bin1,2,*(
), LI Bin1,2,*( )
)
Received:2025-01-21
Online:2025-11-23
Published:2025-11-27
Contact:
YANG Shanshan, WANG Xiaodu, LI Bin
E-mail:1161824097@qq.com;yangshanshan@caas.cn;xdwang@zafu.edu.cn;libinana@126.com
CLC Number:
CAO Qiuxia, YAN Kexin, CHENG Zhenkong, BIAN Xianyu, WANG Chuanhong, LI Sufen, ZHANG Xuehan, FAN Baochao, GUO Rongli, YANG Shanshan, WANG Xiaodu, LI Bin. Expression and Biological Activity Analysis of Porcine CCL25 Recombinant Protein[J]. Acta Veterinaria et Zootechnica Sinica, 2025, 56(11): 5864-5874.
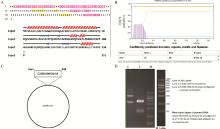
Fig. 1
Construction and identification of the recombinant plasmid vector pCold-SUMO-CCL25 A. Secondary structure prediction of CCL25; B. Prediction of the transmembrane region of CCL25; C. Schematic diagram of the construction of pCold-SUMO-CCL25 recombinant plasmid; D. Verification of the recombinant plasmid by double digestion (M. KB Ladder; 1. pCold-SUMO-CCL25 plasmid; 2. pCold-SUMO-CCL25 plasmid digested by EcoRI and EcoRV)"
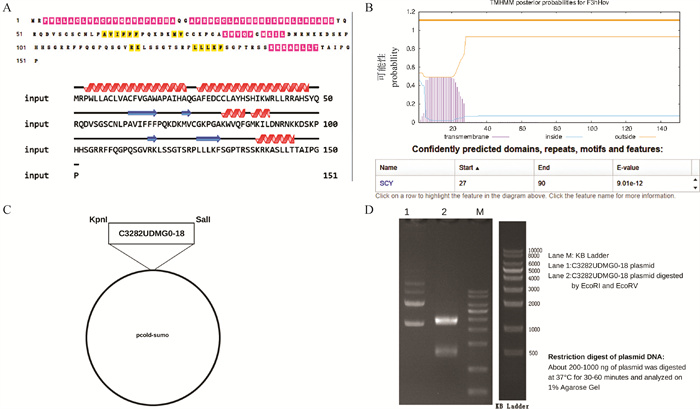
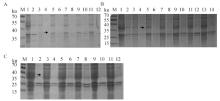
Fig. 2
Optimization of the induction expression conditions of porcine-derived recombinant CCL25 A. Screening at different sampling times (M. Marker; 1.pCold-SUMO supernatant; 2.pCold-SUMO pellet; 3, 5, 7, 9, 11. pCold-SUMO-CCL25 supernatant 8, 12, 16, 20, 24 h sampling; 4, 6, 8, 10, 12. pCold-SUMO-CCL25 pellet at 8, 12, 16, 20, 24 h sampling); B. Screening at different OD600 nm (1. pCold-SUMO supernatant; 2 pCold-SUMO pellet; 3, 5, 7, 9, 11.pCold-SUMO-CCL25 supernat at OD600 nm=0.2, 0.4, 0.6, 0.8, 1.0; 4, 6, 8, 10, 12. pCold-SUMO-CCL25 pellet at OD600 nm=0.2, 0.4, 0.6, 0.8, 1.0, 1.2); C. Screening at different IPTG (M. Marker; 1, 3, 5, 7, 9, 11.pCold-SUMO-CCL25 supernatant at IPTG=0, 0.2, 0.4, 0.6, 0.8, 1.0 mmol ·L-1; 2, 4, 6, 8, 10, 12.pCold-SUMO-CCL25 pellet at IPTG=0, 0.2, 0.4, 0.6, 0.8, 1.0 mmol ·L-1)"
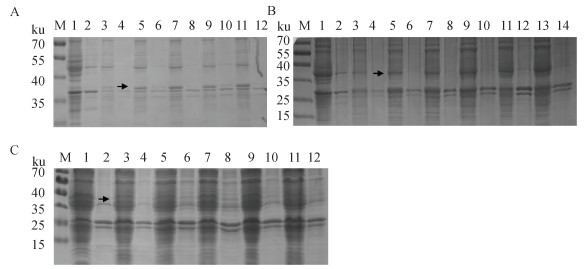
Fig. 3
Purification and identification of porcine CCL25 A.Identification of purified pCold-SUMO-CCL25 by SDS-PAGE (M. Marker; 1. pCold-SUM supernatant; 2. pCold-SUMO pellet; 3. pCold-SUMO-CCL25 supernatant; 4. pCold-SUMO-CCL25 pellet; 5. Purified pCold-SUMO-CCL25 protein); B. Identification of purified pCold-SUMO-CCL25 by Western blot (1. pCold-SUMO; 2. Purified porcine CCL25 protein); C. Purified porcine CCL25 by ELISA"
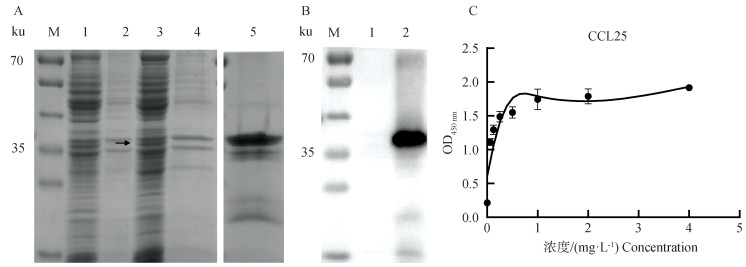
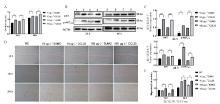
Fig. 4
Functional identification of porcine-derived recombinant CCL25 A.CCK8 assay; B.Western blot (1.5.10 μg ·L-1 SUMO; 2.6.10 μg ·L-1 CCL25; 3.7.100 μg ·L-1 SUMO; 4.8.100 μg ·L-1 CCL25); C.Bar analysis of the gray value of B by ImageJ. D.Scratch assay (Scale bar: 500 μm); E.Bar analysis of the scratch distance of D by ImageJ (ns. No significant difference, *.P < 0.05 **.P < 0.01, ***.P < 0.001)"
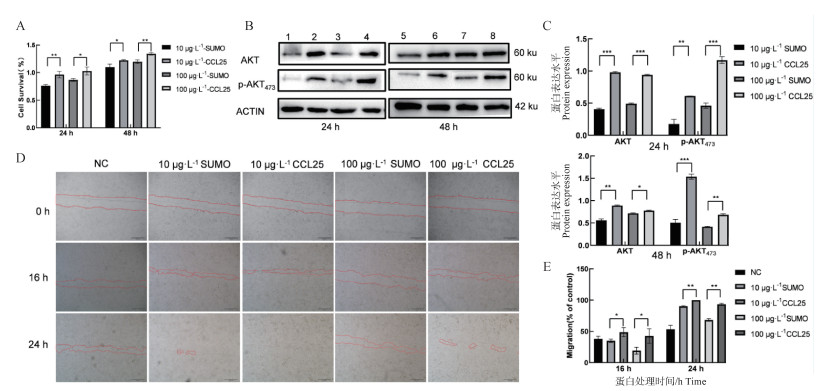

Fig. 5
The effects of CCL25 on cell inflammation, apoptosis and death by RT-qPCR and FCM A.RT-qPCR detection of IL-8 and caspase-3 expression at the transcriptional level in PBMC cells; B.RT-qPCR detection of IL-8 and caspase-3 expression at the transcriptional level in IPEC-J2 cells; C.PBMC flow cytometry 7-AAD expression"
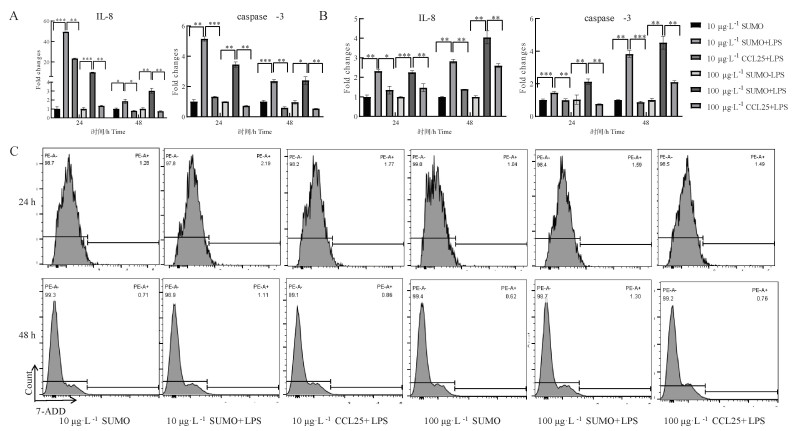

Fig. 6
Detection of CCL25 chemotaxis of PBMC cells using Transwell A. Counting of cells in the lower chamber of the Transwell (scale bar: 500 μm); B. Schematic diagram the operation of the Transwell experiment; C. Histogram analysis of the cell number in A by ImageJ (ns. No significant difference, *. P < 005, **. P<0.01, ***. P < 0.001)"
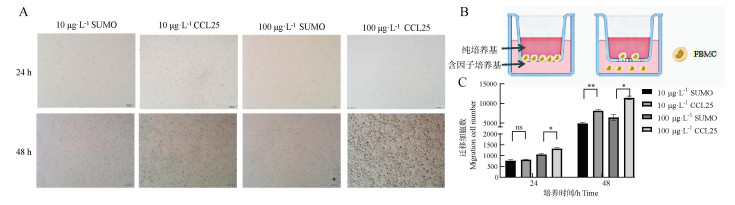
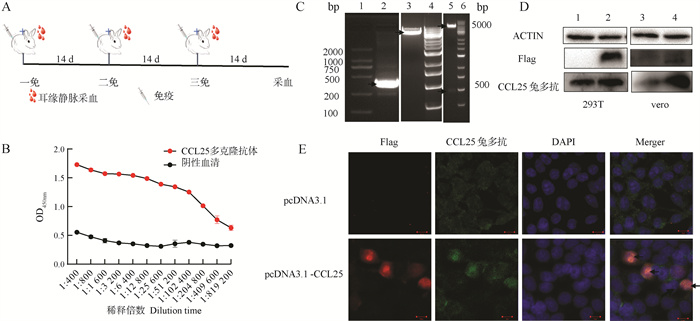
Fig. 7
Preparation and identification of rabbit polyclonal antibody A.Schematic diagram of the immunization process for the rabbit polyclonal antibody; B.ELISA detection of antibody titer; C.Construction and double enzyme digestion identification of pcDNA3.1-CCL25 (1.2 000 DNA marker; 2.PCR amplification product CCL25 gene; 3.Double enzyme digestion of pcDNA3.1 plasmid; 4.6.1 kd marker; 5.pcDNA3.1-CCL25 plasmid digested by EcoRI and XhoⅠ); D.Western blot (1.3.10 μg pcDNA3.1; 2.4.1.0 μg pcDNA3.1-CCL25); E.Conf detection of the co-localization of CCL25 rabbit polyclonal antibody and tag (Scale bar: 10 μm)"
| 1 | 乔新安, 陈丽颖, 王艳玲, 等. CCL25/CCR9和CCL28/CCR10在仔猪胃肠道组织中的表达[J]. 中国农学通报, 2007, 23 (5): 1- 5. |
| QIAO X A , CHEN L Y , WANG Y L , et al. Expression of CCL25/CCR9 and CCL28/CCR10 in the gastrointestinal tract of piglets[J]. Chinese Agricultural Science Bulletin, 2007, 23 (5): 1- 5. | |
| 2 | 钟万锷, 周国雄, 丁晓凌, 等. 趋化因子MIP-3α及其受体CCR6在溃疡性结肠炎中的表达及其意义[J]. 第二军医大学学报, 2008, 29 (10): 1180- 1183. |
| ZHONG W E , ZHOU G X , DING X L , et al. Expression of chemokine MIP-3α and its receptor CCR6 in ulcerative colitis and its significance[J]. Academic Journal of Second Military Medical University, 2008, 29 (10): 1180- 1183. | |
| 3 |
HERNÁNDEZ-RUIZ M , ZLOTNIK A . Mucosal chemokines[J]. J Interferon Cytokine Res, 2017, 37 (2): 62- 70.
doi: 10.1089/jir.2016.0076 |
| 4 | MEURENS F , BERRI M , WHALE J , et al. Expression of TECK/CCL25 and MEC/CCL28 chemokines and their respective receptors CCR9 and CCR10 in porcine mucosal tissues[J]. Vet Immunol Immunopathol, 2006, 113 (3): 313- 327. |
| 5 | WANG C , LIU Z , XU Z , et al. The role of chemokine receptor 9/chemokine ligand 25 signaling: From immune cells to cancer cells[J]. Oncol Lett, 2018, 16 (2): 2071- 2077. |
| 6 | 张婷婷, 王琛, 沈晨, 等. CCL25/CCR9在口腔扁平苔藓外周血的表达及对T细胞功能的影响[J]. 口腔生物医学, 2019, 10 (3): 118- 122. |
| ZHANG T T , WANG C , SHEN C , et al. Expression of CCL25/CCR9 in peripheral blood of patients with oral lichen planus and its effect on T cell function[J]. Oral Biomedicine, 2019, 10 (3): 118- 122. | |
| 7 | 李杰, 桑锋, 刘真, 等. CCL25/CCR9-肠淋巴细胞归巢通路共变化调控肠黏膜免疫屏障损伤与艾滋病从脾论治[J]. 中华中医药学刊, 2023, 41 (2): 89- 92. |
| LI J , SANG F , LIU Z , et al. CCL25/CCR9-intestinal lymphocyte homing pathway co-changes regulate intestinal mucosal immune barr[J]. Chinese Journal of Traditional Chinese Medicine, 2023, 41 (2): 89- 92. | |
| 8 |
PIOVAN E , TOSELLO V , AMADORI A , et al. Chemotactic cues for NOTCH1-dependent leukemia[J]. Front Immunol, 2018, 9, 633.
doi: 10.3389/fimmu.2018.00633 |
| 9 | 沈斌. CCR9/CCL25在NSCLC患者中的表达和促进NSCLC细胞侵袭、迁移作用的研究[D]. 南宁: 广西医科大学, 2017. |
| SHEN B. Study on the expression of CCR9/CCL25 in NSCLC patients and its role in promoting the invasion and migration of NSCLC cells[D]. Nanning: Guangxi Medical University, 2017. (in Chinese) | |
| 10 |
GRISSHAMMER R . Understanding recombinant expression of membrane proteins[J]. Curr Opin Biotechnol, 2006, 17 (4): 337- 340.
doi: 10.1016/j.copbio.2006.06.001 |
| 11 |
JIANG H L , QIN X H , TAO Z , et al. Expression of soluble native protein in Escherichia coli using a cold-shock SUMO tag-fused expression vector[J]. Biotechnol Rep, 2018, 19, e00261.
doi: 10.1016/j.btre.2018.e00261 |
| 12 |
HONG G F , YAN B L , XIU Q Z , et al. Codon optimization with deep learning to enhance protein expression[J]. Sci Rep, 2020, 10 (1): 17617- 17617.
doi: 10.1038/s41598-020-74091-z |
| 13 | 张建楼, 徐瑞涛, 霍珊珊, 等. 猪细小病毒NS1基因的克隆、表达及密码子优化提高表达水平[J]. 中国兽医学报, 2018, 38 (10): 1840- 1845. |
| ZHANG J L , XU R T , HUO S S , et al. Cloning, expression and codon optimization of Porcine parvovirus NS1 gene[J]. Chinese Journal of Veterinary Medicine, 2018, 38 (10): 1840- 1845. | |
| 14 | 董媛, 李汶柯, 孟桂先, 等. 牛妊娠相关糖蛋白18重组载体构建、表达条件优化和生物信息学分析[J]. 中国兽医杂志, 2023, 59 (10): 31- 39. |
| DONG Y , LI W K , MENG G X , et al. Construction of recombinant vector of bovine pregnancy-associated glycoprotein 18, optimization of expression conditions and bioinformatics analysis[J]. Chinese Journal of Veterinary Medicine, 2023, 59 (10): 31- 39. | |
| 15 | NIU Y , TANG D , FAN L , et al. CCL25 promotes the migration and invasion of non-small cell lung cancer cells by regulating VEGF and MMPs in a CCR9-dependent manner[J]. Exp Ther Med, 2020, 19 (6): 3571- 3580. |
| 16 | 李天明, 孙思予, 刘冬妍. 肠道黏膜趋化因子研究进展[J]. 临床军医杂志, 2020, 48 (11): 1380-1382, 1385. |
| LI T M , SUN S Y , LIU D Y . Research progress of intestinal mucosal chemokines[J]. Clinical Journal of Medical Officers, 2020, 48 (11): 1380-1382, 1385. | |
| 17 | 马腾, 刘桂红, 冯芝恩. CCR9及CCL25趋化因子轴在口腔鳞癌转移中的作用[J]. 北京口腔医学, 2022, 30 (2): 98- 101. |
| MA T , LIU G H , FENG Z E . The role of CCR9 and CCL25 chemokine axis in the metastasis of oral squamous cell carcinoma[J]. Beijing Journal of Stomatology, 2022, 30 (2): 98- 101. | |
| 18 | XUE W , MENG S , ZHI Y , et al. The roles of CCR9/CCL25 in inflammation and inflammation-associated diseases[J]. Front Cell Develop Biol, 2021, 19 (9): 686548. |
| 19 | 徐瀚哲, 黄燕. 趋化因子受体9/趋化因子配体25在相关疾病中的研究进展[J]. 中国医药导报, 2023, 20 (35): 37-40, 49. |
| XU H Z , HUANG Y . Advances in chemokine receptor 9 chemokine ligand 25 in related diseases[J]. China Medical Herald, 2023, 20 (35): 37-40, 49. | |
| 20 |
TRIVEDI P J , ADAMS D H . Chemokines and chemokine receptors as therapeutic targets in inflammatory bowel disease; pitfalls and promise[J]. J Crohns Colitis, 2018, 12 (12): 1508.
doi: 10.1093/ecco-jcc/jjy130 |
| 21 |
WANG S , WU C , ZHANG Y , ZHONG Q , et al. Integrin α4β7 switches its ligand specificity via distinct conformer-specific activation[J]. J Cell Biol, 2018, 217 (8): 2799- 2812.
doi: 10.1083/jcb.201710022 |
| 22 |
FENG N , JAIMES M C , LAZARUS N H , et al. Redundant role of chemokines CCL25/TECK and CCL28/MEC in IgA+ plasmablast recruitment to the intestinal lamina propria after rotavirus infection[J]. J Immunol, 2006, 176 (10): 5749- 5759.
doi: 10.4049/jimmunol.176.10.5749 |
| 23 |
HSUEH F C , CHANG Y C , KAO C F , et al. Intramuscular immunization with chemokine-adjuvanted inactive porcine epidemic diarrhea virus induces substantial protection in pigs[J]. Vaccines (Basel), 2020, 8 (1): 102.
doi: 10.3390/vaccines8010102 |
| 24 |
HSU C W , CHANG M H , CHANG H W , et al. Parenterally administered porcine epidemic diarrhea virus-like particle-based vaccine formulated with CCL25/28 chemokines induces systemic and mucosal immune protectivity in pigs[J]. Viruses, 2020, 12 (10): 1122.
doi: 10.3390/v12101122 |
| 25 | 张芹, 丁步坚, 张标. 黏膜免疫佐剂研究进展[J/OL]. 中国免疫学杂志, 2024. (2024-03-21)[2025-09-02]. https://link.cnki.net/urlid/22.1126.R.20240321.1413.002. |
| ZHANG Q, DING B J, ZHANG B. Research progress on mucosal immune adjuvants[J]. Chinese Journal of Immunology, 2024. (2024-03-21)[2025-09-02]. https://link.cnki.net/urlid/22.1126.R.20240321.1413.002. (in Chinese) |
| [1] | GUO Deyang, HU Hui, ZHENG Xueli, JIANG Yanfen. Prokaryotic Expression and Analysis of Bacteriostatic Effects of Porcine β-defensin-1 [J]. Acta Veterinaria et Zootechnica Sinica, 2025, 56(6): 2836-2846. |
| [2] | ZHAO Yunhai, ZHANG Yangyang, MA Haiyun, WANG Qing, HE Xiaoxiao, LIU Kai, ZHANG Yuting, LIU Yudong, YANG Yongning, WU Xiaochun, XING Xiaoyong, QUAN Guomei, ZHANG Zhixiong, BAO Shijun. Prokaryotic Expression and Adhesion Characteristics of Molecular Chaperone Dnak of Mycoplasma bovis [J]. Acta Veterinaria et Zootechnica Sinica, 2025, 56(6): 2868-2878. |
| [3] | WU Qiong, LI Lingdan, YUAN Hui, BIN Chen, DENG Ke, LI Wei, YE Shiyi, LI Guopan, SHEN Qingchun, XIONG Tao. Prokaryotic Expression of PoIFN-α 8s and Identification of Its Activity in vitro and in vivo [J]. Acta Veterinaria et Zootechnica Sinica, 2025, 56(5): 2413-2423. |
| [4] | ZHAO Long, LIN Jingyi, DOU Wei, XU Tingxuan, GU Qingyun, GAO Haihui, LI Shengqing, GUO Kangkang. In vitro Screening of Tibetan Medicine with Inhibitory Effects on Bovine Coronavirus Replication [J]. Acta Veterinaria et Zootechnica Sinica, 2025, 56(2): 826-838. |
| [5] | SHAO Yongheng, NI Minting, GAO Mengling, TANG Jiao, ZHANG Gengxin, LIN Shengyu, LIU Guangliang, CHEN Jianing, WANG Wenhui. Prokaryotic Expression of VP1 Protein to Porcine Teschovirus Type 5 and the Establishment of an Indirect ELISA Detection Method [J]. Acta Veterinaria et Zootechnica Sinica, 2025, 56(2): 883-889. |
| [6] | ZHANG Yaling, JING Wei, ZHAO Yan, HE Xiaobing, FANG Yongxiang, SU Yang, LI Xiaoming, ZHANG Hui, JING Zhizhong, CHEN Guohua. Establishment of Prokaryotic Expression of the ORF002 Protein of Lumpy Skin Disease Virus and an Indirect ELISA Antibody Detection Method [J]. Acta Veterinaria et Zootechnica Sinica, 2025, 56(11): 5817-5825. |
| [7] | ZENG Miaomiao, YANG Xiaoman, ZHANG Xin, LIU Dakai, SHI Hongyan, ZHANG Jiyu, ZHANG Liaoyuan, CHEN Jianfei, FENG Tingshuai, LI Xiuwen, SHI Da, FENG Li. Establishment and Preliminary Application of an Indirect ELISA for Swine Acute Diarrhea Syndrome Coronavirus N Protein [J]. Acta Veterinaria et Zootechnica Sinica, 2025, 56(1): 319-326. |
| [8] | Liguo GAO, Hanqin SHEN, Yiquan CHEN, Sheng CHEN, Wencheng LIN, Feng CHEN. Prokaryotic Expression of Recombinant VP6* Protein of Porcine Rotavirus and Establishment of Indirect ELISA Detection Method [J]. Acta Veterinaria et Zootechnica Sinica, 2024, 55(9): 4021-4028. |
| [9] | Yanxin CHEN, Ruiqi HUA, Guoqing SHAO, Xiaowei ZHU, Wei HOU, Shengqiong LI, Aiguo YANG, Guangyou YANG. Prokaryotic Expression and Secretion Characterization of Annexin B5, B15, and B25 from Echinococcus granulosus [J]. Acta Veterinaria et Zootechnica Sinica, 2024, 55(6): 2607-2618. |
| [10] | SONG Xiaoqing, DENG Ruide, LI Xin, LI Jiao, LI Runcheng, DU Lifei, DONG Wei, GE Meng. Establishment of ELISA for Detection of PCV4-Cap Antibody and Sero-epidemiological Survey [J]. Acta Veterinaria et Zootechnica Sinica, 2024, 55(5): 2072-2079. |
| [11] | LUO Xiaofen, XIE Xiaodong, ZHAO Chao, HU Qian, WANG Yongxuan, RAN Fangfei, HU Pengfei, WEN Ming, ZHU Erpeng, CHENG Zhentao. Initial Identification of Adhesion-related Proteins of Mycoplasma bovis of Guizhou Strains [J]. Acta Veterinaria et Zootechnica Sinica, 2024, 55(4): 1672-1683. |
| [12] | Fukang LIU, Ligang YUAN, Da ZHANG, Aoxing TANG, Guangqing LIU, Jie ZHU. Preparation and Application of N Protein Polyclonal Antibody of Feline Infectious Peritonitis Virus SH2021 Strain [J]. Acta Veterinaria et Zootechnica Sinica, 2024, 55(10): 4773-4778. |
| [13] | XIE Qingyun, XING Huixuan, YU Yanfei, YUAN Ting, XIONG Qiyan, XIONG Fuqiang, FENG Zhixin. Prokaryotic Expression, Polyclonal Antibody Preparation and Activity Identification of Helicase RuvA from Mycoplasma hyopneumoniae [J]. Acta Veterinaria et Zootechnica Sinica, 2024, 55(1): 271-281. |
| [14] | LIU Chuanxia, WANG Xiao, LI Xuewen, BAO Miaofei, LI Tingting, CHEN Xin, WENG Changjiang, ZHENG Jun. Preparation of Monoclonal Antibody of African Swine Fever Virus pE120R [J]. Acta Veterinaria et Zootechnica Sinica, 2024, 55(1): 388-394. |
| [15] | WANG Jingyu, PAN Yangyang, XU Gengquan, ZHANG Rui, ZHANG Wenlan, WANG Xiaoshan, WU Rentaodi, ZHAO Rigetu, CUI Yan, YU Sijiu. Preparation and Preliminary Application of Yak (Bos Grunniens) Fas-associated Factor 1 Polyclonal Antibody [J]. Acta Veterinaria et Zootechnica Sinica, 2023, 54(8): 3369-3382. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||