





Acta Veterinaria et Zootechnica Sinica ›› 2025, Vol. 56 ›› Issue (9): 4215-4231.doi: 10.11843/j.issn.0366-6964.2025.09.008
• Review • Previous Articles Next Articles
BAI Guangdong( ), LOU Zekai, WANG Ruiqi, ZHAO Xuan, LI Jiawei, XIA Yaoyao, PANG Jiaman*(
), LOU Zekai, WANG Ruiqi, ZHAO Xuan, LI Jiawei, XIA Yaoyao, PANG Jiaman*( )
)
Received:2024-10-11
Online:2025-09-23
Published:2025-09-30
Contact:
PANG Jiaman
E-mail:baiguangdong@swu.edu.cn;pangjm@swu.edu.cn
CLC Number:
BAI Guangdong, LOU Zekai, WANG Ruiqi, ZHAO Xuan, LI Jiawei, XIA Yaoyao, PANG Jiaman. Advances in m6A Methylation Modification and Nutritional Regulation in Livestock and Poultry[J]. Acta Veterinaria et Zootechnica Sinica, 2025, 56(9): 4215-4231.
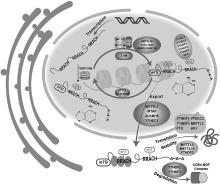
Fig. 1
m6A methyltransferases, m6A demethylases, and m6A reader proteins[6] The m6A methylation modification is a dynamic and reversible process, regulated by m6A methyltransferases, m6A demethylases, and m6A recognition proteins. m6A methyltransferase is responsible for catalysing the transfer of methyl group to adenine of nucleotide RNA molecule to complete the modification; m6A demethylase is responsible for catalysing the methylation of methylated adenine to remove the methyl group; m6A methyl recognition protein is responsible for specifically recognising and binding the protein to achieve the m6A methylation"
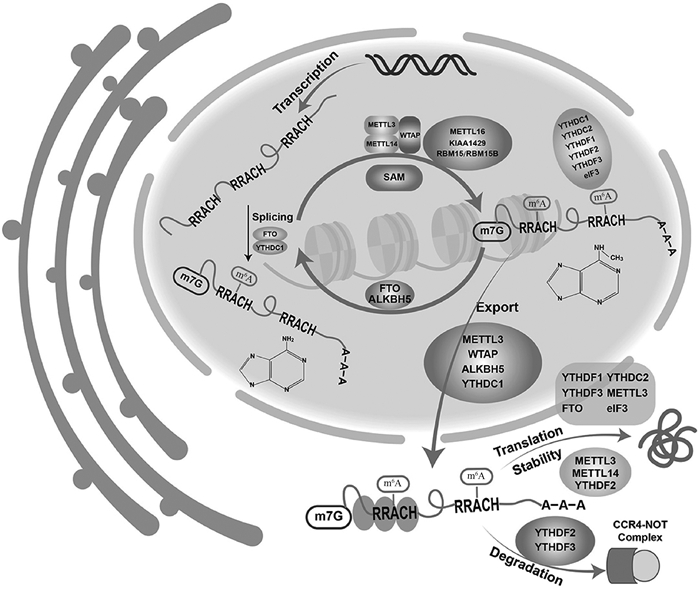
Table 1
Effect of pathogenic bacteria on m6A methylation modification in livestock and poultry"
| 病原性细菌 Pathogenic bacteria | 器官/细胞 Organs/cells | m6A甲基转移酶/甲基化水平 m6A methylase/methylation levels | 文献来源 Literature sources |
| 金黄色葡萄球菌 Staphylococcus aureus | 牛乳腺上皮细胞 | METTL3、METTL14、WTAP、ALKBH5↑ | [ |
| 大肠杆菌 Escherichia coli | 牛乳腺上皮细胞 | METTL3、METTL14、WTAP、ALKBH5↑ | [ |
| 大肠杆菌F18 Escherichia coli F18(E.coli F18) | IPEC-J2 | METTL3↑ | [ |
| IPEC-J2 | METTL3↑ | [ | |
| IPEC-J2 | WTAP↑ | [ | |
| 大肠杆菌K88 Escherichia coli K88(E.coli K88) | IPEC-J2 | m6A水平↑ | [ |
| C型产气荚膜梭菌 Clostridium perfringens beta2 toxin(CPB2) | 仔猪回肠 | METTL3、METTL14、FTO、ALKBH5、YTHDF1↑ WATP、YTHDF2、YTHDF3、YTHDC2↓ m6A水平↑ | [ |
| IPEC-J2 | METTL3、ALKBH5、YTHDF3↑ METTL14、WATP、FTO、YTHDC1、YTHDC2↓ m6A水平↑ | [ | |
| 副猪嗜血杆菌 Haemophilus parasuis(Hps) | 猪肺泡巨噬细胞 | METTL3、METTL14、WTAP、YTHDF2和FTO↓ | [ |
| 1 |
HENGWEI Y , RAZA S H A , WENZHEN Z , et al. Research progress of m6A regulation during animal growth and development[J]. Mol Cell Probes, 2022, 65, 101851.
doi: 10.1016/j.mcp.2022.101851 |
| 2 |
MI S Y , SHI Y J , DARI G , et al. Function of m6A and its regulation of domesticated animals' complex traits[J]. J Anim Sci, 2022, 100 (3): skac034.
doi: 10.1093/jas/skac034 |
| 3 | 张娟丽, 杨姣姣, 杨巧丽, 等. m6A甲基化修饰对畜禽生产影响的研究进展[J]. 中国兽医学报, 2023, 43 (6): 1333- 1341. |
| ZHANG J L , YANG J J , YANG Q L , et al. Research progress of m6a methylation modification of livestock and poultry[J]. Chinese Journal of Veterinary Science, 2023, 43 (6): 1333- 1341. | |
| 4 |
MEYER K D , SALETORE Y , ZUMBO P , et al. Comprehensive analysis of mRNA methylation reveals enrichment in 3' UTRs and near stop codons[J]. Cell, 2012, 149 (7): 1635- 1646.
doi: 10.1016/j.cell.2012.05.003 |
| 5 |
HUANG H , WENG H , CHEN J . m6A modification in coding and non-coding RNAs: roles and therapeutic implications in cancer[J]. Cancer Cell, 2020, 37 (3): 270- 288.
doi: 10.1016/j.ccell.2020.02.004 |
| 6 |
CAI T , ATTEH L L , ZHANG X , et al. The N6-Methyladenosine modification and its role in mRNA metabolism and gastrointestinal tract disease[J]. Front Surg, 2022, 9, 819335.
doi: 10.3389/fsurg.2022.819335 |
| 7 |
SCHÖLLER E , WEICHMANN F , TREIBER T , et al. Interactions, localization, and phosphorylation of the m6A generating METTL3-METTL14-WTAP complex[J]. RNA, 2018, 24 (4): 499- 512.
doi: 10.1261/rna.064063.117 |
| 8 |
BAWANKAR P , LENCE T , PAOLANTONI C , et al. Hakai is required for stabilization of core components of the m6A mRNA methylation machinery[J]. Nat Commun, 2021, 12 (1): 3778.
doi: 10.1038/s41467-021-23892-5 |
| 9 |
KARANDASHOV I K A , DUKICH M , PONOMAREVA N , et al. m6A methylation in regulation of antiviral innate immunity[J]. Viruses, 2024, 16, 601.
doi: 10.3390/v16040601 |
| 10 |
GUIMARAES-TEIXEIRA C , BARROS-SILVA D , LOBO J , et al. Deregulation of N6-methyladenosine RNA modification and its erasers FTO/ALKBH5 among the main renal cell tumor subtypes[J]. J Pers Med, 2021, 11 (10): 996.
doi: 10.3390/jpm11100996 |
| 11 |
JIA G , FU Y , ZHAO X , et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO[J]. Nat Chem Biol, 2011, 7 (12): 885- 887.
doi: 10.1038/nchembio.687 |
| 12 |
KAUR S , TAM N Y , MCDONOUGH M A , et al. Mechanisms of substrate recognition and N6-methyladenosine demethylation revealed by crystal structures of ALKBH5-RNA complexes[J]. Nucleic Acids Res, 2022, 50 (7): 4148- 4160.
doi: 10.1093/nar/gkac195 |
| 13 |
ALARCON C R , GOODARZI H , LEE H , et al. HNRNPA2B1 is a mediator of m6A-dependent nuclear RNA processing events[J]. Cell, 2015, 162 (6): 1299- 1308.
doi: 10.1016/j.cell.2015.08.011 |
| 14 |
HUANG H , WENG H , SUN W , et al. Recognition of RNA N6-methyladenosine by IGF2BP proteins enhances mRNA stability and translation[J]. Nat Cell Biol, 2018, 20 (3): 285- 295.
doi: 10.1038/s41556-018-0045-z |
| 15 |
YU T , QI X , ZHANG L , et al. Dynamic reprogramming and function of RNA N6-methyladenosine modification during porcine early embryonic development[J]. Zygote, 2021, 29 (6): 417- 426.
doi: 10.1017/S0967199420000799 |
| 16 |
COLLIGNON E , CHO B , FURLAN G , et al. m6A RNA methylation orchestrates transcriptional dormancy during paused pluripotency[J]. Nat Cell Biol, 2023, 25 (9): 1279- 1289.
doi: 10.1038/s41556-023-01212-x |
| 17 | 丁浩, 林月月, 张涛, 等. m6A甲基化在鸡肌肉生长发育中的表达研究[J]. 中国畜牧兽医, 2021, 48 (5): 1525- 1534. |
| DING H , LIN Y Y , ZHANG T , et al. Study on the expression of m6A methylation in chicken muscle growth and development[J]. China Animal Husbandry & Veterinary Medicine, 2021, 48 (5): 1525- 1534. | |
| 18 |
ZHANG D , WU S , ZHANG X , et al. Coordinated transcriptional and post-transcriptional epigenetic regulation during skeletal muscle development and growth in pigs[J]. J Anim Sci Biotechnol, 2022, 13 (1): 146.
doi: 10.1186/s40104-022-00791-3 |
| 19 |
GU L , ZHANG S , LI B , et al. m6A and miRNA jointly regulate the development of breast muscles in duck embryonic stages[J]. Front Vet Sci, 2022, 9, 933850.
doi: 10.3389/fvets.2022.933850 |
| 20 |
DANG Y , DONG Q , WU B , et al. global landscape of m6A methylation of differently expressed genes in muscle tissue of Liaoyu white cattle and Simmental cattle[J]. Front Cell Dev Biol, 2022, 10, 840513.
doi: 10.3389/fcell.2022.840513 |
| 21 |
HE S , WANG H , LIU R , et al. mRNA N6-methyladenosine methylation of postnatal liver development in pig[J]. PLoS One, 2017, 12 (3): e0173421.
doi: 10.1371/journal.pone.0173421 |
| 22 |
MA C , CHANG M , LV H , et al. RNA m6A methylation participates in regulation of postnatal development of the mouse cerebellum[J]. Genome Biol, 2018, 19 (1): 68.
doi: 10.1186/s13059-018-1435-z |
| 23 |
ZHOU W , XUE P , YANG Y , et al. Research progress on N6-methyladenosine in the human placenta[J]. J Perinat Med, 2022, 50 (8): 1115- 1123.
doi: 10.1515/jpm-2021-0665 |
| 24 |
WANG S , ZHANG L , XUAN R , et al. Identification and functional analysis of m6A in the mammary gland tissues of dairy goats at the early and peak lactation stages[J]. Front Cell Dev Biol, 2022, 10, 945202.
doi: 10.3389/fcell.2022.945202 |
| 25 |
WANG Y , ZHENG Y , GUO D , et al. m6A methylation analysis of differentially expressed genes in skin tissues of coarse and fine type Liaoning Cashmere goats[J]. Frontiers in Genet, 2020, 10, 1318.
doi: 10.3389/fgene.2019.01318 |
| 26 |
CHEN Y , WANG J , XU D , et al. m6A mRNA methylation regulates testosterone synthesis through modulating autophagy in leydig cells[J]. Autophagy, 2021, 17 (2): 457- 475.
doi: 10.1080/15548627.2020.1720431 |
| 27 |
LIN Z , HSU P J , XING X , et al. METTL3-/METTL14-mediated mRNA N6-methyladenosine modulates murine spermatogenesis[J]. Cell Res, 2017, 27 (10): 1216- 1230.
doi: 10.1038/cr.2017.117 |
| 28 | XUESONG SUI A K, AND LU GAO. RNA m6A modifications in mammalian gametogenesis and pregnancy[J]. Reproduction, 2022, 165(1): R1-R8. |
| 29 |
DU L , LI Y , KANG M , et al. USP48 is upregulated by METTL14 to attenuate hepatocellular carcinoma via regulating SIRT6 stabilization[J]. Cancer Res, 2021, 81 (14): 3822- 3834.
doi: 10.1158/0008-5472.CAN-20-4163 |
| 30 |
LI Y , HE L , WANG Y , et al. N6-methyladenosine methyltransferase KIAA1429 elevates colorectal cancer aerobic glycolysis via HK2-dependent manner[J]. Bioengineered, 2022, 13 (5): 11923- 11932.
doi: 10.1080/21655979.2022.2065952 |
| 31 |
LI H , ZHANG N , JIAO X , et al. Downregulation of microRNA-6125 promotes colorectal cancer growth through YTHDF2-dependent recognition of N6-methyladenosine-modified GSK3β[J]. Clin transl med, 2021, 11 (10): e602.
doi: 10.1002/ctm2.602 |
| 32 |
CHEN B , HONG Y , GUI R , et al. N6-methyladenosine modification of circ_0003215 suppresses the pentose phosphate pathway and malignancy of colorectal cancer through the miR-663b/DLG4/G6PD axis[J]. Cell Death Dis, 2022, 13 (9): 804.
doi: 10.1038/s41419-022-05245-2 |
| 33 |
LIU J , LUO G , SUN J , et al. METTL14 is essential for β-cell survival and insulin secretion[J]. Biochim Biophys Acta Mol Basis Dis, 2019, 1865 (9): 2138- 2148.
doi: 10.1016/j.bbadis.2019.04.011 |
| 34 | 江芹. mRNA m6A修饰关键基因对猪肌内脂肪沉积的影响及机制[D]. 杭州: 浙江大学, 2018. |
| JIANG Q. Effects and mechanisms of key mRNA m6A genes in porcine intramuscular fat deposition[D]. Hangzhou: Zhejiang University, 2018. (in Chinese) | |
| 35 | CHAO M , WANG M , HAN H , et al. Profiling of m6A methylation in porcine intramuscular adipocytes and unravelling PHKG1 represses porcine intramuscular lipid deposition in an m6A-dependent manner[J]. Int J Biol Macromol, 2024, 272 (Pt 1): 132728. |
| 36 |
LUO G , WANG S , AI Y , et al. N6-methyladenosine methylome profiling of muscle and adipose tissues reveals methylase-mRNA metabolic regulatory networks in fat deposition of Rex rabbits[J]. Biology, 2022, 11 (7): 944.
doi: 10.3390/biology11070944 |
| 37 |
LI K , HUANG W , WANG Z , et al. m6A demethylase FTO regulate CTNNB1 to promote adipogenesis of chicken preadipocyte[J]. J Anim Sci Biotechnol, 2022, 13 (1): 147.
doi: 10.1186/s40104-022-00795-z |
| 38 |
韩皓哲, 帖子航, 庞卫军, 等. IGF2BP2介导的m6A修饰调控动物脂肪沉积的研究进展[J]. 畜牧兽医学报, 2023, 54 (9): 3605- 3612.
doi: 10.11843/j.issn.0366-6964.2023.09.002 |
|
HAN H Z , TIE Z H , PANG W J , et al. Advances of IGF2BP2-mediated m6A modification on animal fat deposition[J]. Acta Veterinaria et Zootechnica Sinica, 2023, 54 (9): 3605- 3612.
doi: 10.11843/j.issn.0366-6964.2023.09.002 |
|
| 39 |
DUAN Y , LV X , CAO X , et al. Effect of METTL3 gene on lipopolysaccharide induced damage to primary small intestinal epithelial cells in sheep[J]. Int J Mol Sci, 2024, 25 (17): 9316.
doi: 10.3390/ijms25179316 |
| 40 |
LI S , WANG Y , XU A , et al. Dietary selenomethionine reduced oxidative stress by resisting METTL3-mediated m6A methylation level of Nrf2 to ameliorate LPS-induced liver necroptosis in laying hens[J]. J Nutr Biochem, 2024, 125, 109563.
doi: 10.1016/j.jnutbio.2023.109563 |
| 41 |
XU H , LIN C , WANG C , et al. ALKBH5 stabilized N6-methyladenosine-modified LOC4191 to suppress E.coli-induced apoptosis[J]. Cells, 2023, 12 (22): 2604.
doi: 10.3390/cells12222604 |
| 42 |
FENG Y , SHEN J , LIN Z , et al. PXR activation relieves deoxynivalenol-induced liver oxidative stress via MALAT1 lncRNA m6A demethylation[J]. Adv Sci, 2024, 11 (25): e2308742.
doi: 10.1002/advs.202308742 |
| 43 | YANG J , ZHANG J , GAO X , et al. FTO regulates apoptosis in CPB2-treated IPEC-J2 cells by targeting caspase 3 apoptotic protein[J]. Animals (Basel), 2022, 12 (13): 1644. |
| 44 |
XU H , LIN C , LI T , et al. N6-methyladenosine-modified circRNA in the bovine mammary epithelial cells injured by Staphylococcus aureus and Escherichia coli[J]. Front Immunol, 2022, 13, 873330.
doi: 10.3389/fimmu.2022.873330 |
| 45 | 束婧婷, 单艳菊, 姬改革, 等. 广西麻鸡m6A甲基转移酶基因表达与肌纤维类型及成肌分化的关系[J]. 中国农业科学, 2022, 55 (3): 13. |
| SHU J T , SHAN Y J , JI G G , et al. Relationship between expression levels of Guangxi Partridge chicken m6A Methyltransferase genes, myofiber types and myogenic differentiation[J]. Scientia Agricultura Sinica, 2022, 55 (3): 13. | |
| 46 |
CAO Y , ZHANG S , WANG G , et al. Expression analysis of m6A-related genes in various tissues of Meishan pigs at different developmental stages[J]. R Bra Zootec, 2023, 52, e20210149.
doi: 10.37496/rbz5220210149 |
| 47 | 王明会. m6A修饰在高脂日粮诱导的肉鸡和小鼠糖脂代谢紊乱中的作用[D]. 泰安: 山东农业大学, 2022. |
| WANG M H. Effects of m6A modification in high-fat diet-Induced the disorders of glucose and lipid metabolism in broilers and mice[D]. Taian: Shandong Agricultural University, 2022. (in Chinese) | |
| 48 |
GEBEYEW K , YANG C , MI H , et al. Lipid metabolism and m6A RNA methylation are altered in lambs supplemented rumen-protected methionine and lysine in a low-protein diet[J]. J. Anim Sci Biotechnol, 2022, 13 (1): 85.
doi: 10.1186/s40104-022-00733-z |
| 49 |
CAI S , QUAN S , YANG G , et al. Nutritional status impacts epigenetic regulation in early embryo development: A scoping Review[J]. Adv Nutr, 2021, 12 (5): 1877- 1892.
doi: 10.1093/advances/nmab038 |
| 50 |
SONG T , LU J , DENG Z , et al. Maternal obesity aggravates the abnormality of porcine placenta by increasing N6-methyladenosine[J]. Int J Obes (Lond), 2018, 42 (10): 1812- 1820.
doi: 10.1038/s41366-018-0113-2 |
| 51 | 丰伟丽, 薛智慧, 赵庆博, 等. 呕吐毒素体外刺激3D4/21猪肺泡巨噬细胞对炎性因子和m6A甲基化相关基因表达的影响[J]. 中国畜牧杂志, 2024, 60 (7): 295- 303. |
| FENG W L , XUE Z H , ZHAO Q B , et al. Effect of in vitro stimulation of 3D4/21 porcine alveolar macrophages with DON on the expression of inflammatory factors and m6A methylation-related genes[J]. Chinese Journal of Animal Science, 2024, 60 (7): 295- 303. | |
| 52 | 刘亮, 苏晓彤, 李慧贤, 等. 玉米赤霉烯酮对湖羊睾丸间质细胞毒性及m6A甲基化修饰相关基因表达的影响[J]. 南京农业大学学报, 2023, 46 (4): 772- 779. |
| LIU L , SU X T , LI H X , et al. Effects of zearalenone on cytotoxicity and m6A methylation related gene expression in leydig cells of Hu sheep[J]. Journal of Nanjing Agricultural University, 2023, 46 (4): 772- 779. | |
| 53 |
WU K , JIA S , ZHANG J , et al. Transcriptomics and flow cytometry reveals the cytotoxicity of aflatoxin B1 and aflatoxin M1 in bovine mammary epithelial cells[J]. Ecotoxicol Environ Saf, 2021, 209, 111823.
doi: 10.1016/j.ecoenv.2020.111823 |
| 54 |
DING H , LI Z , LI X , et al. FTO alleviates CdCl2-induced apoptosis and oxidative stress via the AKT/Nrf2 pathway in bovine granulosa cells[J]. Int J Mol Sci, 2022, 23 (9): 4948.
doi: 10.3390/ijms23094948 |
| 55 |
LI W , TAN M , WANG H , et al. METTL3-mediated m6A mRNA modification was involved in cadmium-induced liver injury[J]. Environ Pollut, 2023, 331, 121887.
doi: 10.1016/j.envpol.2023.121887 |
| 56 |
SUN Y , LIU G , LI M , et al. Study on the correlation between regulatory proteins of N6-methyladenosine and oxidative damage in cadmium-induced renal injury[J]. Biol Trace Elem Res, 2023, 201 (5): 2294- 302.
doi: 10.1007/s12011-022-03345-w |
| 57 |
BAI L , TANG Q , ZOU Z , et al. m6A demethylase FTO regulates dopaminergic neurotransmission deficits caused by arsenite[J]. Toxico Sci, 2018, 165 (2): 431- 446.
doi: 10.1093/toxsci/kfy172 |
| 58 |
GU S , SUN D , DAI H , et al. N6-methyladenosine mediates the cellular proliferation and apoptosis via microRNAs in arsenite-transformed cells[J]. Toxico Lett, 2018, 292, 1- 11.
doi: 10.1016/j.toxlet.2018.04.018 |
| 59 |
WANG C , SUN H , JIANG X , et al. Maternal oxidized soybean oil administration in rats during pregnancy and lactation alters the intestinal DNA methylation in offspring[J]. J Agric Food Chem, 2022, 70 (20): 6224- 6238.
doi: 10.1021/acs.jafc.2c01100 |
| 60 |
CAYIR A , BYUN H M , BARROW T M . Environmental epitranscriptomics[J]. Environ Res, 2020, 189, 109885.
doi: 10.1016/j.envres.2020.109885 |
| 61 |
HE F , MU X , ZHANG Y , et al. Late gestational exposure to fenvalerate impacts ovarian reserve in neonatal mice via YTHDF2-mediated P-body assembly[J]. Sci Total Environ, 2024, 925, 171790.
doi: 10.1016/j.scitotenv.2024.171790 |
| 62 |
CHEN N , TANG J , SU Q , et al. Paraquat-induced oxidative stress regulates N6-methyladenosine (m6A) modification of circular RNAs[J]. Environ Pollut, 2021, 290, 117816.
doi: 10.1016/j.envpol.2021.117816 |
| 63 |
WANG C , XU J , LUO S , et al. Parental exposure to environmentally relevant concentrations of bisphenol-A bis(diphenyl phosphate) impairs vascular development in offspring through DNA/RNA methylation-dependent transmission[J]. Environ Sci Technol, 2023, 57 (43): 16176- 16189.
doi: 10.1021/acs.est.3c03579 |
| 64 |
WANG W , LI X , QIAN Q , et al. Mechanistic exploration on neurodevelopmental toxicity induced by upregulation of ALKBH5 targeted by triclosan exposure to larval zebrafish[J]. J Hazard Mater, 2023, 457, 131831.
doi: 10.1016/j.jhazmat.2023.131831 |
| 65 |
QI Y , ZHANG Y , ZHANG J , et al. The alteration of N6-methyladenosine (m6A) modification at the transcriptome-wide level in response of heat stress in bovine mammary epithelial cells[J]. BMC Genomics, 2022, 23 (1): 829.
doi: 10.1186/s12864-022-09067-6 |
| 66 |
LU Z , MA Y , LI Q , et al. The role of N6-methyladenosine RNA methylation in the heat stress response of sheep (Ovis aries)[J]. Cell Stress Chaperones, 2019, 24 (2): 333- 342.
doi: 10.1007/s12192-018-00965-x |
| 67 |
CHEN B , YUAN C , GUO T , et al. Molecular mechanism of m6A methylation modification genes METTL3 and FTO in regulating heat stress in sheep[J]. Int J Mol Sci, 2023, 24 (15): 11926.
doi: 10.3390/ijms241511926 |
| 68 |
李笑微, 田微, 刘媛, 等. 高温应激下湖羊卵巢颗粒细胞m6A甲基化修饰差异研究[J]. 畜牧兽医学报, 2025, 56 (4): 1712- 1721.
doi: 10.11843/j.issn.0366-6964.2025.04.020 |
|
LI X W , TIAN W , LIU Y , et al. Study on the difference of m6A methylation modification in ovarian granulosa cells of Hu Sheep under heat stress[J]. Acta Veterinaria et Zootechnica Sinica, 2025, 56 (4): 1712- 1721.
doi: 10.11843/j.issn.0366-6964.2025.04.020 |
|
| 69 | KISLIOUK T, ROSENBERG T, BEN-NUN O, et al. Early-life m6A RNA demethylation by fat mass and obesity-associated protein (FTO) influences resilience or vulnerability to heat stress later in life[J]. eNeuro, 2020, 7(3): ENEURO. 0549-19.2020. |
| 70 |
SUN M H , JIANG W J , LI X H , et al. High temperature-induced m6A epigenetic changes affect early porcine embryonic developmental competence in pigs[J]. Microsc Microanal, 2023, 29 (6): 2174- 2183.
doi: 10.1093/micmic/ozad131 |
| 71 |
HENG J , TIAN M , ZHANG W , et al. Maternal heat stress regulates the early fat deposition partly through modification of m6A RNA methylation in neonatal piglets[J]. Cell Stress Chaperones, 2019, 24 (3): 635- 645.
doi: 10.1007/s12192-019-01002-1 |
| 72 |
CAYIR A , BARROW T M , GUO L , et al. Exposure to environmental toxicants reduces global N6-methyladenosine RNA methylation and alters expression of RNA methylation modulator genes[J]. Environ Res, 2019, 175, 228- 234.
doi: 10.1016/j.envres.2019.05.011 |
| 73 |
JI C , TAO Y , LI X , et al. AHR-mediated m6A RNA methylation contributes to PM2.5-induced cardiac malformations in zebrafish larvae[J]. J Hazard Mater, 2023, 457, 131749.
doi: 10.1016/j.jhazmat.2023.131749 |
| 74 |
HE X , ZHANG L , LIU S , et al. Methyltransferase-like 3 leads to lung injury by up-regulation of interleukin 24 through N6-methyladenosine-dependent mRNA stability and translation efficiency in mice exposed to fine particulate matter 2.5[J]. Environ Pollut, 2022, 308, 119607.
doi: 10.1016/j.envpol.2022.119607 |
| 75 |
XING Y , TANG Y , CHEN Q , et al. The role of RNA epigenetic modification-related genes in the immune response of cattle to mastitis induced by Staphylococcus aureus[J]. Anim Biosci, 2024, 37 (7): 1141- 1155.
doi: 10.5713/ab.23.0323 |
| 76 | 李婷. RNA m6A修饰调控金黄色葡萄球菌及大肠杆菌诱导的奶牛乳腺上皮细胞炎症反应机制[D]. 武汉: 华中农业大学, 2021. |
| LI T. RNA Regulatory mechanism of RNA m6A modification on inflammatory response of bovine mammary epithelial cells induced by Staphylococcus aureus/Escherichia coli[D]. Wuhan: Huazhong Agricultural University, 2021. (in Chinese) | |
| 77 | JIN J , LIU M , YU F , et al. METTL3 enhances E. coli F18 resistance by targeting IKBKG/NF-κB signaling via an m6A-YTHDF1-dependent manner in IPEC-J2 cells[J]. Int J Biol Macromol, 2024, 262 (Pt 2): 130101. |
| 78 | 王诗琴. METTL3介导m6A修饰对断奶仔猪F18大肠杆菌抗性的调控机制分析[D]. 扬州: 扬州大学, 2021. |
| WANG S Q. Analysis of the regulatory mechanism of METTL3-mediated m6A modification on E. coli F18 resistance in weaned piglets[D]Yangzhou: Yangzhou University, 2021. (in Chinese) | |
| 79 |
WU Z , WANG Y , LI T , et al. Comprehensive analysis revealed the potential roles of N6-methyladenosine (m6A) mediating E. coli F18 susceptibility in IPEC-J2 Cells[J]. Int J Mol Sci, 2022, 23 (21): 13602.
doi: 10.3390/ijms232113602 |
| 80 |
ZONG X , WANG H , XIAO X , et al. Enterotoxigenic Escherichia coli infection promotes enteric defensin expression via FOXO6-METTL3-m6A-GPR161 signalling axis[J]. RNA Biol, 2021, 18 (4): 576- 586.
doi: 10.1080/15476286.2020.1820193 |
| 81 | 张娟丽. m6A修饰在CPB2毒素诱导猪IPEC-J2细胞损伤中的功能机制研究[D]. 兰州: 甘肃农业大学, 2022. |
| ZHANG J L. Functional mechanism of m6A modification in CPB2 toxin-indued injury of porcine IPEC-J2 cells[D]. Lanzhou: Gansu Agricultural University, 2022. (in Chinese) | |
| 82 | 薛智慧, 郭鑫, 李水莲, 等. 副猪嗜血杆菌体外刺激3D4/21猪肺泡巨噬细胞对炎性因子和m6A相关基因表达的影响[J]. 黑龙江畜牧兽医, 2022 (7): 71- 75. |
| XUE Z H , GUO X , LI S L , et al. Effect of in vitro stimulation of Haemophilus parasuis on the expression of inflammatory factors and m6A-related genes in porcine alveolar macrophage 3D4 /21 cells[J]. Heilongjiang Animal Science and Veterinary Medicine, 2022 (7): 71- 75. | |
| 83 |
ZHANG J , YANG J , GAO X , et al. METTL3 regulates the inflammatory response in CPB2 toxin-exposed IPEC-J2 cells through the TLR2/NF-κB signaling pathway[J]. Int J Mol Sci, 2022, 23 (24): 15833.
doi: 10.3390/ijms232415833 |
| 84 |
GONG X , LIANG Y , WANG J , et al. Highly pathogenic PRRSV upregulates IL-13 production through nonstructural protein 9-mediated inhibition of N6-methyladenosine demethylase FTO[J]. J Biol Chem, 2024, 300 (4): 107199.
doi: 10.1016/j.jbc.2024.107199 |
| 85 |
CHEN J , SONG H X , HU J H , et al. Classical swine fever virus non-structural protein 5B hijacks host METTL14-mediated m6A modification to counteract host antiviral immune response[J]. PLoS Pathog, 2024, 20 (3): e1012130.
doi: 10.1371/journal.ppat.1012130 |
| 86 |
JIN J , XU C , WU S , et al. m6A demethylase ALKBH5 restrains PEDV infection by regulating GAS6 expression in porcine alveolar macrophages[J]. Int J Mol Sci, 2022, 23 (11): 6191.
doi: 10.3390/ijms23116191 |
| 87 |
LI C , WU X , YANG Y , et al. METTL14 and FTO mediated m6A modification regulate PCV2 replication by affecting miR-30a-5p maturity[J]. Virulence, 2023, 14 (1): 2232910.
doi: 10.1080/21505594.2023.2232910 |
| 88 |
NABI KHAN R I , PRAHARAJ M R , MALLA W A , et al. Changes in m6A RNA methylation of goat lung following PPRV infection[J]. Heliyon, 2023, 9 (9): e19358.
doi: 10.1016/j.heliyon.2023.e19358 |
| 89 |
WU L , QUAN W , ZHANG Y , et al. Attenuated duck hepatitis A virus infection is associated with high mRNA maintenance in duckling liver via m6A modification[J]. Front Immunol, 2022, 13, 839677.
doi: 10.3389/fimmu.2022.839677 |
| 90 | WU Y C , LIU X , WANG J L , et al. Soft-shelled turtle peptide modulates microRNA profile in human gastric cancer AGS cells[J]. Oncol Lett, 2018, 15 (3): 3109- 3120. |
| 91 |
FU R , CHEN D , TIAN G , et al. Betaine affects abdominal flare fat metabolism via regulating m6A RNA methylation in finishing pigs fed a low-energy diet[J]. J Funct Foods, 2023, 107, 105620.
doi: 10.1016/j.jff.2023.105620 |
| 92 |
KANG H , ZHANG Z , YU L , et al. FTO reduces mitochondria and promotes hepatic fat accumulation through RNA demethylation[J]. J Cell Biochem, 2018, 119 (7): 5676- 5685.
doi: 10.1002/jcb.26746 |
| 93 |
WANG Y K , YU X X , LIU Y H , et al. Reduced nucleic acid methylation impairs meiotic maturation and developmental potency of pig oocytes[J]. Theriogenology, 2018, 121, 160- 167.
doi: 10.1016/j.theriogenology.2018.08.009 |
| 94 |
LI J , FANG L , XI M , et al. Toxic effects of triclosan on hepatic and intestinal lipid accumulation in zebrafish via regulation of m6A-RNA methylation[J]. Aquat Toxicol, 2024, 269, 106884.
doi: 10.1016/j.aquatox.2024.106884 |
| 95 |
LI N , ZHANG D , CAO S , et al. The effects of folic acid on RNA m6A methylation in hippocampus as well as learning and memory ability of rats with acute lead exposure[J]. J Funct Foods, 2021, 76, 104276.
doi: 10.1016/j.jff.2020.104276 |
| 96 |
LU N , LI X , YU J , et al. Curcumin attenuates lipopolysaccharide-induced hepatic lipid metabolism disorder by modification of m6A RNA methylation in piglets[J]. Lipids, 2018, 53 (1): 53- 63.
doi: 10.1002/lipd.12023 |
| 97 | 卓儒浩. m6A RNA甲基化介导猪轮状病毒在肠道中感染的机制及姜黄素的营养调控研究[D]. 南京: 南京农业大学, 2022. |
| ZHUO R H. The mechanism of m6A RNA methylation mediating porcine rotavirus infection in intestine and the nutritional regulation of curcumin[D]. Nanjing: Nanjing Agricultural University, 2022. (in Chinese) | |
| 98 |
CHEN Y , WU R , CHEN W , et al. Curcumin prevents obesity by targeting TRAF4-induced ubiquitylation in m6A-dependent manner[J]. EMBO Rep, 2021, 22 (5): e52146.
doi: 10.15252/embr.202052146 |
| 99 |
GAN Z , WEI W , WU J , et al. Resveratrol and curcumin improve intestinal mucosal integrity and decrease m6A RNA methylation in the intestine of weaning piglets[J]. ACS Omega, 2019, 4 (17): 17438- 17446.
doi: 10.1021/acsomega.9b02236 |
| 100 | QIAO Y , XIAO G , ZHU X , et al. Resveratrol enhances antioxidant and anti-apoptotic capacities in chicken primordial germ cells through m6A methylation: A preliminary investigation[J]. Animals (Basel), 2024, 14 (15): 2214. |
| 101 |
WU J , GAN Z , ZHUO R , et al. Resveratrol attenuates aflatoxin B1-induced ROS formation and increase of m6A RNA methylation[J]. Animals, 2020, 10 (4): 677.
doi: 10.3390/ani10040677 |
| 102 |
ZHANG M , LIU J , YU C , et al. Berberine regulation of cellular oxidative stress, apoptosis and autophagy by modulation of m6A mRNA methylation through targeting the Camk1db/ERK pathway in zebrafish-hepatocytes[J]. Antioxidants (Basel), 2022, 11 (12): 2370.
doi: 10.3390/antiox11122370 |
| 103 |
LIU R , WANG Q , LI Y , et al. Ginsenoside Rg1 alleviates sepsis-induced acute lung injury by reducing FBXO3 stability in an m6A-dependent manner to activate PGC-1α/Nrf2 signaling pathway[J]. Aaps j, 2024, 26 (3): 47.
doi: 10.1208/s12248-024-00919-5 |
| 104 | 韩笑, 王梦斐, 蒲位凌, 等. 中药活性成分通过调节m6A甲基化修饰抗肿瘤的研究进展[J]. 中草药, 2024, 55 (6): 2123- 2130. |
| HAN X , WANG M F , PU W L , et al. Advances in anti-cancer research of active ingredients of traditional Chinese medicine through regulation of m6A methylation modification[J]. Chinese Traditional and Herbal Drugs, 2024, 55 (6): 2123- 2130. | |
| 105 |
LI T , TAN Y-T , CHEN Y-X , et al. Methionine deficiency facilitates antitumour immunity by altering m6A methylation of immune checkpoint transcripts[J]. Gut, 2023, 72 (3): 501- 511.
doi: 10.1136/gutjnl-2022-326928 |
| 106 |
SHIMA H , MATSUMOTO M , ISHIGAMI Y , et al. S-Adenosylmethionine synthesis is regulated by selective N6-Adenosine methylation and mRNA degradation involving METTL16 and YTHDC1[J]. Cell Rep, 2017, 21 (12): 3354- 3363.
doi: 10.1016/j.celrep.2017.11.092 |
| 107 | HENG J , WU Z , TIAN M , et al. Excessive BCAA regulates fat metabolism partially through the modification of m6A RNA methylation in weanling piglets[J]. Nutr Metab(Lond), 2020, 17, 10. |
| 108 |
WANG Z , CAI D , LI K , et al. Transcriptome analysis of the inhibitory effect of cycloleucine on myogenesis[J]. Poult Sci, 2022, 101 (12): 102219.
doi: 10.1016/j.psj.2022.102219 |
| 109 |
ZHANG M , ZHANG S , ZHAI Y , et al. Cycloleucine negatively regulates porcine oocyte maturation and embryo development by modulating N6-methyladenosine and histone modifications[J]. Theriogenology, 2022, 179, 128- 140.
doi: 10.1016/j.theriogenology.2021.11.024 |
| 110 |
YANG Y W , CHEN L , MOU Q , et al. Ascorbic acid promotes the reproductive function of porcine immature Sertoli cells through transcriptome reprogramming[J]. Theriogenology, 2020, 158, 309- 320.
doi: 10.1016/j.theriogenology.2020.09.022 |
| 111 |
YU X X , LIU Y H , LIU X M , et al. Ascorbic acid induces global epigenetic reprogramming to promote meiotic maturation and developmental competence of porcine oocytes[J]. Sci Rep, 2018, 8 (1): 6132.
doi: 10.1038/s41598-018-24395-y |
| 112 | 靳艾. RNA m6A修饰在LPS诱导的肺部炎症中的作用机制及维生素D3的缓解作用[D]. 杨凌: 西北农林科技大学, 2022. |
| JIN A. The effect and mechanism of RNA N6-methyladenosine modification in LPS-induced pulmonary inflammation and the effect of vitamin D3 on its mitigation[D]. Yangling: Northwest A&F University, 2022. (in Chinese) | |
| 113 |
MOSCA P , ROBERT A , ALBERTO J M , et al. Vitamin B12 deficiency dysregulates m6A mRNA methylation of genes involved in neurological functions[J]. Mol Nutr Food Res, 2021, 65 (17): e2100206.
doi: 10.1002/mnfr.202100206 |
| 114 |
WANG S , LU H , SU M , et al. Bisphenol H exposure disrupts Leydig cell function in adult rats via oxidative stress-mediated m6A modifications: Implications for reproductive toxicity[J]. Ecotoxicol Environ Saf, 2024, 285, 117061.
doi: 10.1016/j.ecoenv.2024.117061 |
| 115 |
LIU K , HE X , HUANG J , et al. Short-chain fatty acid-butyric acid ameliorates granulosa cells inflammation through regulating METTL3-mediated N6-methyladenosine modification of FOSL2 in polycystic ovarian syndrome[J]. Clin Epigenetics, 2023, 15 (1): 86.
doi: 10.1186/s13148-023-01487-9 |
| 116 |
CHEN W , CHEN Y , WU R , et al. DHA alleviates diet-induced skeletal muscle fiber remodeling via FTO/m6A/DDIT4/PGC1α signaling[J]. BMC Biology, 2022, 20 (1): 39.
doi: 10.1186/s12915-022-01239-w |
| 117 |
LI Y , MA B , WANG Z , et al. The effect mechanism of N6-adenosine methylation (m6A) in melatonin regulated LPS-induced colon inflammation[J]. Int J Biol Sci, 2024, 20 (7): 2491- 2506.
doi: 10.7150/ijbs.95316 |
| 118 |
HU C , JI F , LV R , et al. Putrescine promotes MMP9-induced angiogenesis in skeletal muscle through hydrogen peroxide/METTL3 pathway[J]. Free Radic Biol Med, 2024, 212, 433- 447.
doi: 10.1016/j.freeradbiomed.2023.12.041 |
| 119 |
GAO J , LI Y , LIU Z , et al. Acetaminophen changes the RNA m6A levels and m6A-related proteins expression in IL-1β-treated chondrocyte cells[J]. BMC Mol Cell Biol, 2022, 23 (1): 45.
doi: 10.1186/s12860-022-00444-3 |
| [1] | YANG Xin, WANG Shaoyu, TONG Chang, PENG Zhitao, CAI Shenghuang, HUANG Junxiong, XU Jiaojiao, WEN Xin, WU Yinbao. Research Progress on the Horizontal Gene Transfer of Antibiotic Resistance Genes from Livestock and Poultry Manure [J]. Acta Veterinaria et Zootechnica Sinica, 2025, 56(9): 4279-4293. |
| [2] | ZHANG Fan, ZENG Wei, ZHOU Ao. Advances in Gene Editing for Disease Resistance Breeding in Livestock and Poultry [J]. Acta Veterinaria et Zootechnica Sinica, 2025, 56(7): 3047-3056. |
| [3] | LI Xiaowei, TIAN Wei, LIU Yuan, LI Huixia. Study on the Difference of m6A Methylation Modification in Ovarian Granulosa Cells of Hu Sheep under Heat Stress [J]. Acta Veterinaria et Zootechnica Sinica, 2025, 56(4): 1712-1721. |
| [4] | WANG Zhuo, ZHAO Yuwei, TU Yan, DIAO Qiyu, CUI Kai. Research Progress on Biological Characteristics of β-defensins and Their Roles in Regulating Intestinal Barrier in Animals [J]. Acta Veterinaria et Zootechnica Sinica, 2025, 56(3): 995-1005. |
| [5] | CHANG Xuan, WEI Bingni, ZHANG Xiaoli, ZHAO Zhongquan, CHEN Juncai. Research Progress of Gastrointestinal Symbiotic Fungi in Livestock and Poultry [J]. Acta Veterinaria et Zootechnica Sinica, 2025, 56(1): 63-73. |
| [6] | Jinbu WANG, Jia LI, Deming REN, Lixian WANG, Ligang WANG. Progress in the Application of Machine Learning in Livestock and Poultry Genomic Selection [J]. Acta Veterinaria et Zootechnica Sinica, 2024, 55(7): 2775-2785. |
| [7] | WANG Yaxin, WANG Jing, TIAN Xuekai, YANG Gongshe, YU Taiyong. Application of Multi-omics Technology in the Study of Important Economic Traits of Livestock and Poultry [J]. Acta Veterinaria et Zootechnica Sinica, 2024, 55(5): 1842-1853. |
| [8] | LI Ke, WANG Yulong, LI Dong, SHI Xin'e, YANG Gongshe, YU Taiyong. Advances in Pan-genome Study of Livestock and Poultry [J]. Acta Veterinaria et Zootechnica Sinica, 2023, 54(9): 3595-3604. |
| [9] | XIA Chunqiu, WAN Fachun, LIU Lei, SHEN Weijun, XIAO Dingfu. Valine: Biological Function and Application in Livestock and Poultry Diets [J]. Acta Veterinaria et Zootechnica Sinica, 2023, 54(11): 4502-4513. |
| [10] | GUO Haikang, WAN Fachun, SHEN Weijun, WANG Zuo. Research Progress and Related Regulation Technology on Bacterial Quorum Sensing in the Gastro-intestinal Tract of Livestock and Poultry [J]. Acta Veterinaria et Zootechnica Sinica, 2022, 53(6): 1678-1688. |
| [11] | SHU Ze, WANG Lixian, WANG Ligang. Research Progress of Alternative Splicing and Its Application in Livestock and Poultry Breeding [J]. Acta Veterinaria et Zootechnica Sinica, 2020, 51(12): 2911-2920. |
| [12] | HU Jing-jie,GUO Xin,LI Ren-wei,GONG Dao-qing,DU Sheng-ming. Pathogenesis Study of Important Pathogens in Livestock and Poultry:An Introduction to a Major Project of National Natural Science Foundation of China [J]. ACTA VETERINARIA ET ZOOTECHNICA SINICA, 2014, 45(12): 2088-2090. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||