





Acta Veterinaria et Zootechnica Sinica ›› 2025, Vol. 56 ›› Issue (8): 3722-3733.doi: 10.11843/j.issn.0366-6964.2025.08.015
• Animal Genetics and Breeding • Previous Articles Next Articles
HU Jinling1( ), ZHONG Qiqi1, HUANG Cheng1, LEI Minggang1,2,3,*(
), ZHONG Qiqi1, HUANG Cheng1, LEI Minggang1,2,3,*( )
)
Received:2025-01-15
Online:2025-08-23
Published:2025-08-28
Contact:
LEI Minggang
E-mail:jinling_hu@163.com;leimg@mail.hzau.edu.cn
CLC Number:
HU Jinling, ZHONG Qiqi, HUANG Cheng, LEI Minggang. AKR1B1 Regulates Proliferation and Differentiation of Porcine Skeletal Muscle Satellite Cells via the AMPK/mTOR/S6 Signaling Pathway[J]. Acta Veterinaria et Zootechnica Sinica, 2025, 56(8): 3722-3733.
Table 1
Mainly used antibodies"
| 名称 Name | 来源 Source | 稀释比 Dilution ratio | 货号 Identifier |
| AKR1B1 Rabbit mAb | Abclonal | 1∶5 000 | A22132 |
| β-Tubulin Mouse mAb | Abclonal | 1∶5 000 | AC021 |
| HRP-conjugated PCNA Monoclonal antibody | Proteintech | 1∶5 000 | 10D10E11 |
| Rabbit Polyclonal Anti-MYH3 | Proteintech | 1∶1 000 | 22287-1-AP |
| mTOR Monoclonal antibody | Proteintech | 1∶5 000 | 81670-1-RR |
| Phospho-mTOR (Ser2448) Recombinant antibody | Proteintech | 1∶5 000 | 67778-1-Ig |
| AMPK Alpha Polyclonal antibody | Proteintech | 1∶5 000 | 10929-2-AP |
| Phospho-AMPK Beta 1 (Ser182) Recombinant antibody | Proteintech | 1∶5 000 | 83924-1-RR |
| S6 Ribosomal protein Recombinant antibody | Proteintech | 1∶5 000 | 80208-1-RR |
| p70(S6K) Polyclonal antibody | Proteintech | 1∶5 000 | 14485-1-AP |
| Tuberin/TSC2 Polyclonal antibody | Proteintech | 1∶5 000 | 24601-1-AP |
| Raptor Polyclonal antibody | Proteintech | 1∶5 000 | 20984-1-AP |
| Rabbit Monoclonal Anti-Cyclin D1 | Proteintech | 1∶5 000 | 60186-1-lg |
| Mouse Polyclonal Anti-Myogenin | Santa | 1∶1 000 | sc-52903 |
| Rabbit Monoclonal Anti-Ki67 | Abmart | 1∶1 000 | TW0001 |
| HRP-conjugated Affnipure Goat Anti-Rabbit lgG (H+L) | Proteintech | 1∶5 000 | SA00001-1 |
| HRP-conjugated Affnipure Goat Anti-Mouse lgG (H+L) | Proteintech | 1∶5 000 | SA00001-2 |
| Cy3 Goat Anti-Rabbit lgG (H+L) | Abclonal | 1∶200 | AS007 |
| Cy3 Goat Anti-Mouse lgG (H+L) | Abclonal | 1∶200 | AS008 |
Table 2
qRT-PCR primers information"
| 名称 Name | 引物序列(5′→3′) Primer sequence | 产物长度/bp Product length |
| sus-AKR1B1 | F: GAGGAGCTCCGCAGTCATGG;R: GTTCTCGGCAATGCGTTCAG | 176 |
| sus-PCNA | F: ATCUGGTGTGACCCGGACT;R: CTGGCATCACCGAAGAGCAGTT | 163 |
| sus-Ki67 | F: AGCCCGTATCTGGTGCAAAA;R: CCTGCATCTGTGTAAGGGCA | 267 |
| sus-ccnd1 | F: ATCAGGTGTGACCCGGACT;R: CGCCTCAAATGTTCACGTCG | 184 |
| sus-MyH3 | F: GAAGAGTACGCCAAGGGGAAA;R: GTGTACCGGTCCTTGAGGTT | 200 |
| sus-MyOG | F: AGGCTACGAGCGGACTGA;R: GCAGGGTGCTCCTCTTCA | 230 |
| sus-GADPH | F: ACCCAGAAGACTGTGGATGG;R: AAGCAGGGATGATGTTCTGG | 79 |
Table 3
Amino acid composition of AKR1B1 protein"
| 氨基酸 Amino acid | 氨基酸数量/个 Number | 占比/% Percent |
| 丙氨酸Ala (A) | 18 | 57 |
| 精氨酸Arg (R) | 10 | 3.2 |
| 天冬氨酸Asn (N) | 15 | 4.7 |
| 天门冬氨酸Asp (D) | 18 | 5.7 |
| 半胱氨酸Cys (C) | 6 | 1.9 |
| 谷氨酰胺Gln (Q) | 11 | 3.5 |
| 谷氨酸Glu (E) | 24 | 7.6 |
| 甘氨酸Gly (G) | 18 | 5.7 |
| 组氨酸His (H) | 9 | 2.8 |
| 异亮氨酸Ile (I) | 16 | 5.1 |
| 丙氨酸Ala (A) | 18 | 57 |
| 精氨酸Arg (R) | 10 | 3.2 |
| 亮氨酸Leu (L) | 34 | 10.8 |
| 赖氨酸Lys (K) | 26 | 8.2 |
| 苯丙氨酸Phe (F) | 10 | 3.2 |
| 甲硫氨酸Met (M) | 6 | 1.9 |
| 脯氨酸Pro (P) | 22 | 7.0 |
| 丝氨酸Ser (S) | 14 | 4.4 |
| 苏氨酸Thr (T) | 14 | 4.4 |
| 色氨酸Trp (W) | 6 | 1.9 |
| 酪氨酸Tyr (Y) | 13 | 4.1 |
| 缬氨酸Val (V) | 26 | 8.2 |

Fig. 1
Composition, structure, and physicochemical properties of AKR1B1 protein A. Hydrophobicity prediction of AKR1B1 protein; B. Signal peptide prediction of AKR1B1 protein; C. Prediction of potential phosphorylation sites of AKR1B1 protein; D. Prediction of the tertiary structure of AKR1B1 protein; E. Prediction of the secondary structure of AKR1B1 protein, among them, the blue line represents the α-helix, the red line represents the extended strand, the purple line represents the random coil"
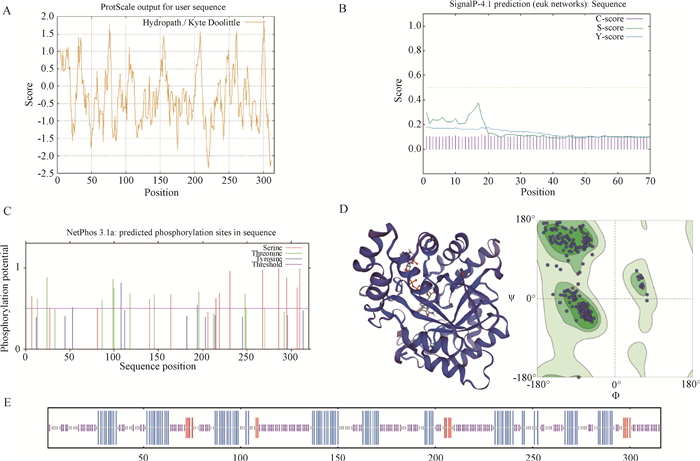
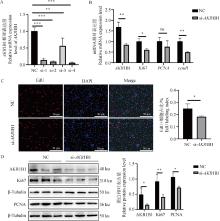
Fig. 3
Effect of AKR1B1 knockdown on the proliferation of PSCs A. Screening of AKR1B1 interference fragments; B. qRT-PCR detection of Ki67, PCNA, and AKR1B1 mRNA expression levels after AKR1B1 interference; C. EdU cell proliferation staining assay after AKR1B1 interference, scale bar: 50 μm; D. Western blot analysis of Ki67, PCNA, and AKR1B1 protein expression levels after AKR1B1 interference. * Indicates significant difference (P < 0.05), ** Indicates highly significant difference (P < 0.01), the same as below"

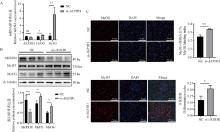
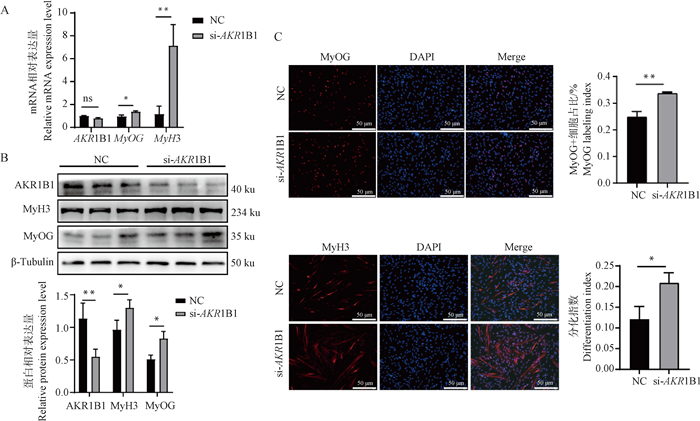
Fig. 4
Effect of AKR1B1 knockdown on the differentiation of PSCs A. qRT-PCR analysis of MyOG, MyH3, and AKR1B1 mRNA expression levels after AKR1B1 interference; B. Western blot analysis of MyOG, MyH3, and AKR1B1 protein expression levels after AKR1B1 interference; C. Immunofluorescence detection of MyOG and MyH3 expression levels and distribution after AKR1B1 interference, scale bar: 50 μm"
| 1 |
PENG J Y , HAN L L , LIU B , et al. Gli1 marks a sentinel muscle stem cell population for muscle regeneration[J]. Nat Commun, 2023, 14 (1): 6993.
doi: 10.1038/s41467-023-42837-8 |
| 2 |
POWNALL M E , GUSTAFSSON M K , EMERSON C P JR . Myogenic regulatory factors and the specification of muscle progenitors in vertebrate embryos[J]. Annu Rev Cell Dev Biol, 2002, 18, 747- 783.
doi: 10.1146/annurev.cellbio.18.012502.105758 |
| 3 |
GUO Q , LUO Q , SONG G B . Control of muscle satellite cell function by specific exercise-induced cytokines and their applications in muscle maintenance[J]. J Cachexia Sarcopenia Muscle, 2024, 15 (2): 466- 476.
doi: 10.1002/jcsm.13440 |
| 4 |
YOSHIMOTO Y , OISHI Y . Mechanisms of skeletal muscle-tendon development and regeneration/healing as potential therapeutic targets[J]. Pharmacol Ther, 2023, 243, 108357.
doi: 10.1016/j.pharmthera.2023.108357 |
| 5 | JEZ J M , BENNETT M J , SCHLEGEL B P , et al. Comparative anatomy of the aldo-keto reductase superfamily[J]. Biochem J, 1997, 326 (Pt 3): 625- 636. |
| 6 |
PETRASH J M . All in the family: Aldose reductase and closely related aldo-keto reductases[J]. Cell Mol Life Sci, 2004, 61 (7-8): 737- 749.
doi: 10.1007/s00018-003-3402-3 |
| 7 | LIU L J , ZHU L H , CHENG Z W , et al. Aberrant expression of akr1b1 indicates poor prognosis and promotes gastric cancer progression by regulating the akt-mtor pathway[J]. Aging (Albany NY), 2023, 15 (18): 9661- 9675. |
| 8 |
ZHANG S Q , YUNG K L K , CHUNG S K , et al. Aldo-keto reductases-mediated cytotoxicity of 2-deoxyglucose: A novel anticancer mechanism[J]. Cancer Sci, 2018, 109 (6): 1970- 1980.
doi: 10.1111/cas.13604 |
| 9 | TANAWATTANASUNTORN T , RATTANABUREE T , THONGPANCHANG T , et al. Trans-(±)-kusunokinin suppresses akr1b1: Inhibition of oxidative stress and alteration of epithelial-mesenchymal transition markers on aggressive cancer[J]. Eur J Cancer, 2022, 174, S71- S72. |
| 10 |
ZHU X P , YAO T , WANG R , et al. Irf4 in skeletal muscle regulates exercise capacity via ptg/glycogen pathway[J]. Adv Sci (Weinh), 2020, 7 (19): 2001502.
doi: 10.1002/advs.202001502 |
| 11 |
BHAGAVATI S , SONG X , SIDDIQUI M A . Rnai inhibition of pax3/7 expression leads to markedly decreased expression of muscle determination genes[J]. Mol Cell Biochem, 2007, 302 (1-2): 257- 262.
doi: 10.1007/s11010-007-9444-3 |
| 12 | BENTZINGER C F , WANG Y X , RUDNICKI M A . Building muscle: Molecular regulation of myogenesis[J]. Cold Spring Harb Perspect Biol, 2012, 4 (2): a008342. |
| 13 |
SARTORE S , GORZA L , SCHIAFFINO S . Fetal myosin heavy chains in regenerating muscle[J]. Nature, 1982, 298 (5871): 294- 296.
doi: 10.1038/298294a0 |
| 14 |
RUIZ F X , PARÉS X , FARRÉS J . Perspective on the structural basis for human aldo-keto reductase 1b10 inhibition[J]. Metabolites, 2021, 11 (12): 865.
doi: 10.3390/metabo11120865 |
| 15 |
BRESSON E , BOUCHER-KOVALIK S , CHAPDELAINE P , et al. The human aldose reductase akr1b1 qualifies as the primary prostaglandin f synthase in the endometrium[J]. J Clin Endocrinol Metab, 2011, 96 (1): 210- 219.
doi: 10.1210/jc.2010-1589 |
| 16 |
MATEO-OTERO Y , RIBAS-MAYNOU J , DELGADO-BERMÚDEZ A , et al. Aldose reductase b1 in pig sperm is related to their function and fertilizing ability[J]. Front Endocrinol (Lausanne), 2022, 13, 773249.
doi: 10.3389/fendo.2022.773249 |
| 17 |
MATEO-OTERO Y , VIÑOLAS-VERGÉIS E , LLAVANERA M , et al. Aldose reductase b1 in pig seminal plasma: Identification, localization in reproductive tissues, and relationship with quality and sperm preservation[J]. Front Cell Dev Biol, 2021, 9, 683199.
doi: 10.3389/fcell.2021.683199 |
| 18 |
CHOI Y , JANG H , SEO H , et al. Changes in calcium levels in the endometrium throughout pregnancy and the role of calcium on endometrial gene expression at the time of conceptus implantation in pigs[J]. Mol Reprod Dev, 2019, 86 (7): 883- 895.
doi: 10.1002/mrd.23166 |
| 19 |
TAO X , LIANG Y , YANG X M , et al. Transcriptomic profiling in muscle and adipose tissue identifies genes related to growth and lipid deposition[J]. PLoS One, 2017, 12 (9): e0184120.
doi: 10.1371/journal.pone.0184120 |
| 20 |
KANG T T , XING W K , XI Y , et al. Mir-543 regulates myoblast proliferation and differentiation of c2c12 cells by targeting[J]. J Cell Biochem, 2020, 121 (12): 4827- 4837.
doi: 10.1002/jcb.29710 |
| 21 |
员佳乐, 刘畅, 黄晓宇, 等. Mir-145-5p靶向igf1r介导akt通路抑制猪骨骼肌卫星细胞增殖和分化[J]. 畜牧兽医学报, 2023, 54 (5): 1893- 1904.
doi: 10.11843/j.issn.0366-6964.2023.05.012 |
|
YUAN J L , LIU C , HUANG X Y , et al. miR-145-5p inhibits the proliferation and differentiation of porcine skeletal muscle satellite cells by targeting IGF1R-mediated AKT pathway[J]. Acta Veterinaria et Zootechnica Sinica, 2023, 54 (5): 1893- 1904.
doi: 10.11843/j.issn.0366-6964.2023.05.012 |
|
| 22 |
JIN C L , YE J L , YANG J Z , et al. Mtorc1 mediates lysine-induced satellite cell activation to promote skeletal muscle growth[J]. Cells, 2019, 8 (12): 1549.
doi: 10.3390/cells8121549 |
| 23 |
WANG S C , TIAN B , FENG X Y , et al. Selenium promotes broiler myoblast proliferation through the ros/pten/pi3k/ akt signaling axis[J]. Poult Sci, 2024, 103 (12): 104364.
doi: 10.1016/j.psj.2024.104364 |
| 24 | CHEN X L , LUO Y L , HUANG Z Q , et al. Akirin2 regulates proliferation and differentiation of porcine skeletal muscle satellite cells via erk1/2 and nfatc1 signaling pathways[J]. Sci Rep, 2017, 7, 45156. |
| 25 |
SAKAMOTO K , FURUICHI Y , YAMAMOTO M , et al. R3hdml regulates satellite cell proliferation and differentiation[J]. EMBO Rep, 2019, 20 (11): e47957.
doi: 10.15252/embr.201947957 |
| 26 | CHEN X Q , CHEN C , HAO J , et al. Akr1b1 upregulation contributes to neuroinflammation and astrocytes proliferation by regulating the energy metabolism in rat spinal cord injury[J]. Neurochem Res, 2018, 43 (8): 1491- 1499. |
| 27 | XIAO M B , JIN D D , JIAO Y J , et al. Β2-ar regulates the expression of akr1b1 in human pancreatic cancer cells and promotes their proliferation via the erk1/2 pathway[J]. Mol Biol Rep, 2018, 45 (6): 1863- 1871. |
| 28 | WU J Y , YUE B L . Regulation of myogenic cell proliferation and differentiation during mammalian skeletal myogenesis[J]. Biomed Pharm, 2024, 174, 116563. |
| 29 |
郑琪, 睢梦华, 凌英会. 骨骼肌卫星细胞增殖与成肌分化过程中关键信号通路的作用[J]. 畜牧兽医学报, 2017, 48 (11): 2005- 2014.
doi: 10.11843/j.issn.0366-6964.2017.11.001 |
|
ZHENG Q , SUI M H , LING Y H . The role of key signaling pathways in the proliferation and differentiation of skeletal muscle satellite cells[J]. Acta Veterinaria et Zootechnica Sinica, 2017, 48 (11): 2005- 2014.
doi: 10.11843/j.issn.0366-6964.2017.11.001 |
|
| 30 | DELFINI M C , HIRSINGER E , POURQUIÉ O , et al. Delta 1-activated notch inhibits muscle differentiation without affecting myf5 and pax3 expression in chick limb myogenesis[J]. Development, 2000, 127 (23): 5213- 5224. |
| 31 | STEINBERG G R , HARDIE D G . New insights into activation and function of the ampk[J]. Nat Rev Mol Cell Biol, 2023, 24 (4): 255- 272. |
| 32 | PAL P B , SONOWAL H , SHUKLA K , et al. Aldose reductase regulates hyperglycemia-induced huvec death via sirt1/ampk-α1/mtor pathway[J]. J Mol Endocrinol, 2019, 63 (1): 11- 25. |
| 33 | WU T T , CHEN Y Y , CHANG H Y , et al. Akr1b1-induced epithelial-mesenchymal transition mediated by rage-oxidative stress in diabetic cataract lens[J]. Antioxidants (Basel), 2020, 9 (4): 273. |
| 34 | FRANKISH B P , MURPHY R M . Does ampk bind glycogen in skeletal muscle or is the relationship correlative[J]. Essays Biochem, 2024, 68 (3): 337- 347. |
| 35 | KISSOW J , JACOBSEN K J , GUNNARSSON T P , et al. Effects of follicular and luteal phase-based menstrual cycle resistance training on muscle strength and mass[J]. Sports Med, 2022, 52 (12): 2813- 2819. |
| 36 | MENG Z T , ZHOU D , LV D , et al. Human milk extracellular vesicles enhance muscle growth and physical performance of immature mice associating with akt/mtor/p70s6k signaling pathway[J]. J Nanobiotechnol, 2023, 21 (1): 304. |
| 37 | OHANNA M , SOBERING A K , LAPOINTE T , et al. Atrophy of s6k1(-/-) skeletal muscle cells reveals distinct mtor effectors for cell cycle and size control[J]. Nat Cell Biol, 2005, 7 (3): 286- 294. |
| 38 | FANG Y , LIANG F , YUAN R Q , et al. High mobility group box 2 regulates skeletal muscle development through ribosomal protein s6 kinase 1[J]. Faseb J, 2020, 34 (9): 12367- 12378. |
| [1] | Xiaojuan LIANG, Yushuang LI, Yingying LI, Shouwei WANG. Isolation, Culture and Adipogenic Differentiation of Beijing Black Pig Preadipocytes [J]. Acta Veterinaria et Zootechnica Sinica, 2024, 55(7): 2877-2889. |
| [2] | WEN Shu-lei, SUN Jia-jie, CHEN Ting, WU Jia-han, SHU Gang, WANG Song-bo, WANG Li-na, JIANG Qing-yan, ZHANG Yong-liang, XI Qian-yun. Research Progress in the Mechanism of Cross-talk between Muscle and Adipose Tissues in Pig [J]. ACTA VETERINARIA ET ZOOTECHNICA SINICA, 2018, 49(12): 2543-2549. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||