





Acta Veterinaria et Zootechnica Sinica ›› 2025, Vol. 56 ›› Issue (2): 803-813.doi: 10.11843/j.issn.0366-6964.2025.02.029
• Preventive Veterinary Medicine • Previous Articles Next Articles
LU Na( ), GAO Yu, ZHAO Jiawei, SU Di, CHEN Jialei, LUO Zhongli*(
), GAO Yu, ZHAO Jiawei, SU Di, CHEN Jialei, LUO Zhongli*( )
)
Received:2024-04-01
Online:2025-02-23
Published:2025-02-26
Contact:
LUO Zhongli
E-mail:lunar@stu.cqmu.edu.cn;Zhongliluo@163.com
CLC Number:
LU Na, GAO Yu, ZHAO Jiawei, SU Di, CHEN Jialei, LUO Zhongli. Construction and Characterization of Transcription Vectors for Feline Infectious Peritonitis Virus mRNA Vaccines[J]. Acta Veterinaria et Zootechnica Sinica, 2025, 56(2): 803-813.
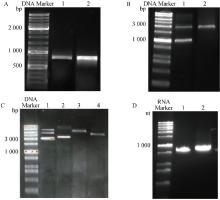
Fig. 3
Construction and identification of recombinant plasmids A. In vitro amplification of the EGFP gene, purified agarose gel after double digestion (lane 1: in vitro amplification of the EGFP gene, lane 2: purification after double digestion of the EGFP gene); B. Agarose gel electropherogram of double digestion of the pBluescript Ⅱ KS(+)-FIPV N recombinant plasmid (lane 1), agarose gel electropherogram of gel-recovered mRNA transcription vector in vitro (lane 2); C. Extraction of recombinant plasmids and linearized agarose gel plots of single digestion (Lane 1: pBluscript lI KS(+)-EGFP plasmid; Lane 2: pBluscript Ⅰ KS(+)-FIPV N plasmid; Lane 3: Single enzyme digested pBluscript Ⅱ KS(+)-FIPV; Lane 4: Single enzyme cleaved pBluscript Il KS(+)-EGFP); D. In vitro transcribed EGFP-mRNA and FIPV N-mRNA agarose gel plots (lane 1:EGFP-mRNA; lane 2:FIPV N-mRNA)"
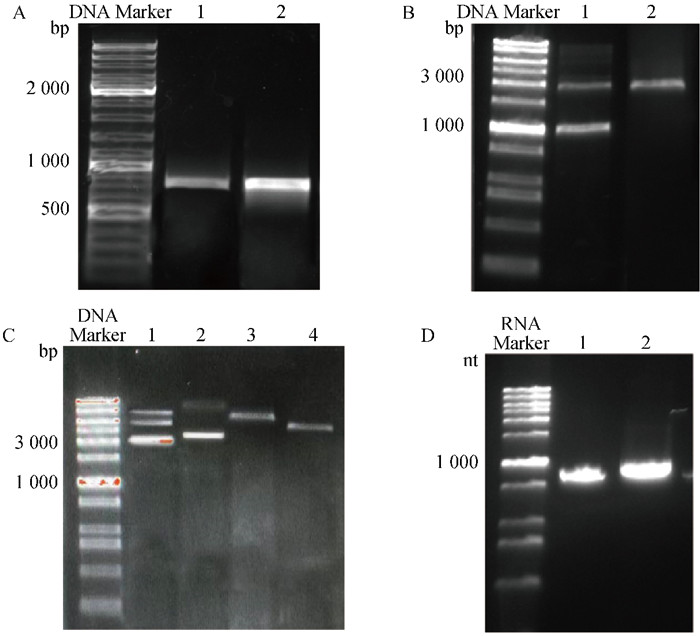

Fig. 5
Expression of FIPV N protein in 293T cells among different trement groups M. Standard protein molecular weight; 1. Negative control group without transfection of FIPV N-mRNA; 2. Expression of FIPV N protein 12 hours after transfection of FIPV N-mRNA using Lipofectamine 3 000; 3. Expression of FIPV N protein 12 hours after transfection of FIPV N-mRNA using Lipofectamine 3 000"


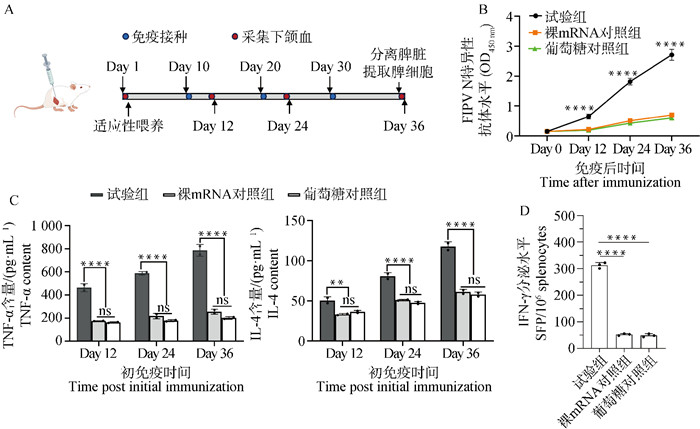
Fig. 7
The immune response elicited by FIPV N-mRNA vaccine in mice after immunization A. Animal experimental immunology flow chart; B. Serum levels of FIPV N-specific antibodies in the grouped mice during immunization; C. TNF-α and IL-4 levels in the serum of the grouped mice during immunization; D. Elispot assay was used to detect the secretion level of IFN-γ factor in mouse splenocytes in each group. ** represents P < 0.01, with statistical difference, **** represents P < 0.000 1, which means significant statistical difference, ns indicates no statistical difference"
| 1 | 李春华, 于瑞嵩, 周宗清. 犬猫冠状病毒病[J]. 上海畜牧兽医通讯, 2023, (2): 1- 9. |
| LI C H , YU R C , ZHOU Z Q . Coronavirus diseases of dogs and cats[J]. Shanghai Journal of Animal Husbandry and Veterinary Medicine, 2023, (2): 1- 9. | |
| 2 |
GAO Y Y , WANG Q , LIANG X Y , et al. An updated review of feline coronavirus: mind the two biotypes[J]. Virus Res, 2023, 326, 199059.
doi: 10.1016/j.virusres.2023.199059 |
| 3 |
DELAPLACE M , HUET H , GAMBINO A , et al. Feline coronavirus antivirals: a review[J]. Pathogens, 2021, 10 (9): 1150.
doi: 10.3390/pathogens10091150 |
| 4 |
GERBER J D , INGERSOLL J D , GAST A M , et al. Protection against feline infectious peritonitis by intranasal inoculation of a temperature-sensitive FIPV vaccine[J]. Vaccine, 1990, 8 (6): 536- 542.
doi: 10.1016/0264-410X(90)90004-6 |
| 5 |
SCHERK M A , FORD R B , GASKELL R M , et al. 2013 AAFP feline vaccination advisory panel report[J]. J Feline Med Surg, 2013, 15 (9): 785- 808.
doi: 10.1177/1098612X13500429 |
| 6 |
TAKANO T , NAKAGUCHI M , DOKI T , et al. Antibody-dependent enhancement of serotype Ⅱ feline enteric coronavirus infection in primary feline monocytes[J]. Arch Virol, 2017, 162 (11): 3339- 3345.
doi: 10.1007/s00705-017-3489-8 |
| 7 | 李双星, 刘业兵, 柳方远, 等. 猫传染性腹膜炎病毒N蛋白在昆虫细胞-杆状病毒系统中的表达与鉴定[J]. 中国畜牧兽医, 2021, 48 (1): 273- 279. |
| LI S X , LIU Y B , LIU F Y , et al. Expression and identification of feline infectious peritonitis virus N protein in insect cell-baculovirus expression system[J]. China Animal Husbandry & Veterinary Medicine, 2021, 48 (1): 273- 279. | |
| 8 |
BÁLINT A , FARSANG A , SZEREDI L , et al. Recombinant feline coronaviruses as vaccine candidates confer protection in SPF but not in conventional cats[J]. Vet Microbiol, 2014, 169 (3-4): 154- 162.
doi: 10.1016/j.vetmic.2013.10.015 |
| 9 |
WANG Y H , LIU Y , WANG J N , et al. An adenovirus-vectored vaccine based on the N protein of feline coronavirus elicit robust protective immune responses[J]. Antiviral Res, 2024, 223, 105825.
doi: 10.1016/j.antiviral.2024.105825 |
| 10 |
HOHDATSU T , YAMATO H , OHKAWA T , et al. Vaccine efficacy of a cell lysate with recombinant baculovirus-expressed feline infectious peritonitis (FIP) virus nucleocapsid protein against progression of FIP[J]. Vet Microbiol, 2003, 97 (1-2): 31- 44.
doi: 10.1016/j.vetmic.2003.09.016 |
| 11 |
MOHSENI N , ROYSTER A , REN S Y , et al. A novel compound targets the feline infectious peritonitis virus nucleocapsid protein and inhibits viral replication in cell culture[J]. J Biol Chem, 2023, 299 (3): 102976.
doi: 10.1016/j.jbc.2023.102976 |
| 12 |
马继炎, 刘佐坤, 黄旸木. 疫苗平台技术的国际发展及对我国新发传染病战略储备的启示[J]. 中国新药杂志, 2023, 32 (10): 1007- 1012.
doi: 10.3969/j.issn.1003-3734.2023.10.007 |
|
MA J Y , LIU Z K , HUANG Y M . International development of vaccine platform technology and its implications for China's strategic stockpile of emerging infectious diseases[J]. Chinese Journal of New Drugs, 2023, 32 (10): 1007- 1012.
doi: 10.3969/j.issn.1003-3734.2023.10.007 |
|
| 13 | 马莉莉, 王琼娴, 王晓丽, 等. 基于杆状病毒Bac-to-Bac系统的VLP递送mRNA疫苗平台的建立[J]. 中国动物传染病学报, 2023. [2024-12-04]. https://doi.org/10.19958/j.cnki.cn31-2031/s.20230726.002. |
| MA L L, WANG Q X, WANG X L, et al. Establishment of a VLP delivery mRNA vaccine platform based on baculovirus Bac-To-Bac system[J]. Chinese Journal of Animal Infectious Diseases, 2023. [2024-12-04]. https://doi.org/10.19958/j.cnki.cn31-2031/s.20230726.002. (in Chinese) | |
| 14 |
GEBRE M S , RAUCH S , ROTH N , et al. Optimization of non-coding regions for a non-modified mRNA COVID-19 vaccine[J]. Nature, 2022, 601 (7893): 410- 414.
doi: 10.1038/s41586-021-04231-6 |
| 15 |
徐小千. 图说mRNA疫苗[J]. 生物学教学, 2022, 47 (6): 84- 88.
doi: 10.3969/j.issn.1004-7549.2022.06.034 |
|
XU X Q . Illustrated with mRNA vaccines[J]. Biology Teaching, 2022, 47 (6): 84- 88.
doi: 10.3969/j.issn.1004-7549.2022.06.034 |
|
| 16 |
KIPAR A , MELI M L . Feline infectious peritonitis: still an enigma?[J]. Vet Pathol, 2014, 51 (2): 505- 526.
doi: 10.1177/0300985814522077 |
| 17 |
GAO Y Y , LIANG X Y , WANG Q , et al. Mind the feline coronavirus: comparison with SARS-CoV-2[J]. Gene, 2022, 825, 146443.
doi: 10.1016/j.gene.2022.146443 |
| 18 | 高嘉淇, 赵献军, 华进联. mRNA疫苗在人和动物重大疫病防控中的研究进展[J]. 生理学报, 2023, 75 (5): 647- 658. |
| GAO J Q , ZHAO X J , HUA J L . Progress on mRNA vaccine for the prevention of major infectious diseases in humans and animals[J]. Acta Physiologica Sinica, 2023, 75 (5): 647- 658. | |
| 19 | 田颖, 张娜娜, 秦成峰, 等. mRNA技术应对病毒传染病的研究进展[J]. 微生物学通报, 2022, 49 (7): 2849- 2861. |
| TIAN Y , ZHANG N N , QIN C F , et al. Advancements in mRNA technology-based therapies for infectious diseases[J]. Microbiology China, 2022, 49 (7): 2849- 2861. | |
| 20 | GERBER J D, PFEIFFER N E, INGERSOLL J D, et al. Characterization of an attenuated temperature sensitive feline infectious peritonitis vaccine virus[M]//CAVANAGH D, BROWN T D K. Coronaviruses and their Diseases. Boston: Springer, 1990: 481-489. |
| 21 |
TAKANO T , TOMIZAWA K , MORIOKA H , et al. Evaluation of protective efficacy of the synthetic peptide vaccine containing the T-helper 1 epitope with CpG oligodeoxynucleotide against feline infectious peritonitis virus infection in cats[J]. Antivir Ther, 2014, 19 (7): 645- 650.
doi: 10.3851/IMP2735 |
| 22 |
STODDART C A , BARLOUGH J E , BALDWIN C A , et al. Attempted immunisation of cats against feline infectious peritonitis using canine coronavirus[J]. Res Vet Sci, 1988, 45 (3): 383- 388.
doi: 10.1016/S0034-5288(18)30970-6 |
| 23 |
HOHDATSU T , YAMADA M , TOMINAGA R , et al. Antibody-dependent enhancement of feline infectious peritonitis virus infection in feline alveolar macrophages and human monocyte cell line U937 by serum of cats experimentally or naturally infected with feline coronavirus[J]. J Vet Med Sci, 1998, 60 (1): 49- 55.
doi: 10.1292/jvms.60.49 |
| 24 |
WANG Z , DENG T T , ZHANG Y L , et al. ACE2 can act as the secondary receptor in the FcγR-dependent ADE of SARS-CoV-2 infection[J]. iScience, 2022, 25 (1): 103720.
doi: 10.1016/j.isci.2021.103720 |
| 25 | WASMOEN T L , KADAKIA N P , UNFER R C , et al. Protection of cats from infectious peritonitis by vaccination with a recombinant raccoon poxvirus expressing the nucleocapsid gene of feline infectious peritonitis virus[J]. Adv Exp Med Biol, 1995, 380, 221- 228. |
| 26 |
ANIS E A , WILKES R P , KANIA S A , et al. Effect of small interfering RNAs on in vitro replication and gene expression of feline coronavirus[J]. Am J Vet Res, 2014, 75 (9): 828- 834.
doi: 10.2460/ajvr.75.9.828 |
| 27 |
PEDERSEN N C . A review of feline infectious peritonitis virus infection: 1963-2008[J]. J Feline Med Surg, 2009, 11 (4): 225- 258.
doi: 10.1016/j.jfms.2008.09.008 |
| 28 | PEDERSEN N C . Virologic and immunologic aspects of feline infectious peritonitis virus infection[J]. Adv Exp Med Biol, 1987, 218, 529- 550. |
| 29 | 秦凤铭, 任宁, 成温玉, 等. 传染病mRNA疫苗: 原理及应用[J]. 生物工程学报, 2023, 39 (10): 3966- 3984. |
| QIN F M , REN N , CHENG W Y , et al. mRNA vaccines for infectious diseases: principles and applications[J]. Chinese Journal of Biotechnology, 2023, 39 (10): 3966- 3984. | |
| 30 |
陈鑫, 秦彤. mRNA疫苗及其在动物传染病中的研究展望[J]. 畜牧兽医学报, 2023, 54 (7): 2732- 2742.
doi: 10.11843/j.issn.0366-6964.2023.07.007 |
|
CHEN X , QIN T . mRNA vaccine and its research prospect in zoonotic diseases[J]. Acta Veterinaria et Zootechnica Sinica, 2023, 54 (7): 2732- 2742.
doi: 10.11843/j.issn.0366-6964.2023.07.007 |
| [1] | Fukang LIU, Ligang YUAN, Da ZHANG, Aoxing TANG, Guangqing LIU, Jie ZHU. Preparation and Application of N Protein Polyclonal Antibody of Feline Infectious Peritonitis Virus SH2021 Strain [J]. Acta Veterinaria et Zootechnica Sinica, 2024, 55(10): 4773-4778. |
| [2] | CAO Liyan, KONG Xiangyu, LI Xiangtong, SUO Xuepeng, DUAN Yueyue, YUAN Cong, SHI Lei, ZHANG Yu, MA Guoxiang, ZHENG Haixue, WANG Qi. Preparation and Sequence Analysis of Monoclonal Antibody against the Nucleocapsid Protein of Porcine Acute Diarrhea Syndrome Coronavirus [J]. Acta Veterinaria et Zootechnica Sinica, 2023, 54(6): 2487-2497. |
| [3] | ZHENG Min, LUO Le, XIONG Chao-li, WU Liang-tao, HUA Min, WAN Run, CHENG Zhen-tao, ZHOU Bi-jun, YANG Qi, WEN Ming. Effection of Targeting DEV-NP Gene on DEV Proliferation by RNA Interference [J]. ACTA VETERINARIA ET ZOOTECHNICA SINICA, 2017, 48(1): 132-139. |
| [4] | TAN Fei-fei;ZHANG Rong;WEI Zu-zhang;YUAN Shi-shan. Translational Control of Nucleocapsid Protein of Porcine Reproductive and Respiratory Syndrome Virus [J]. ACTA VETERINARIA ET ZOOTECHNICA SINICA, 2010, 41(7): 847-853. |
| [5] | LV Mao-jie;FENG Li;SHI Hong-yan;CHEN Jian-fei;SUN Dong-bo;SHEN Shi-chuan;CUI Xiao-chen;WANG Cheng-bao;FAN Xiu-ping;. Eukaryotic Expression and Subcellular Localization Preliminary Analysis of Porcine Epidemic Diarrhea Virus Nucleocapsid Protein [J]. ACTA VETERINARIA ET ZOOTECHNICA SINICA, 2009, 40(5): 691-696. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||